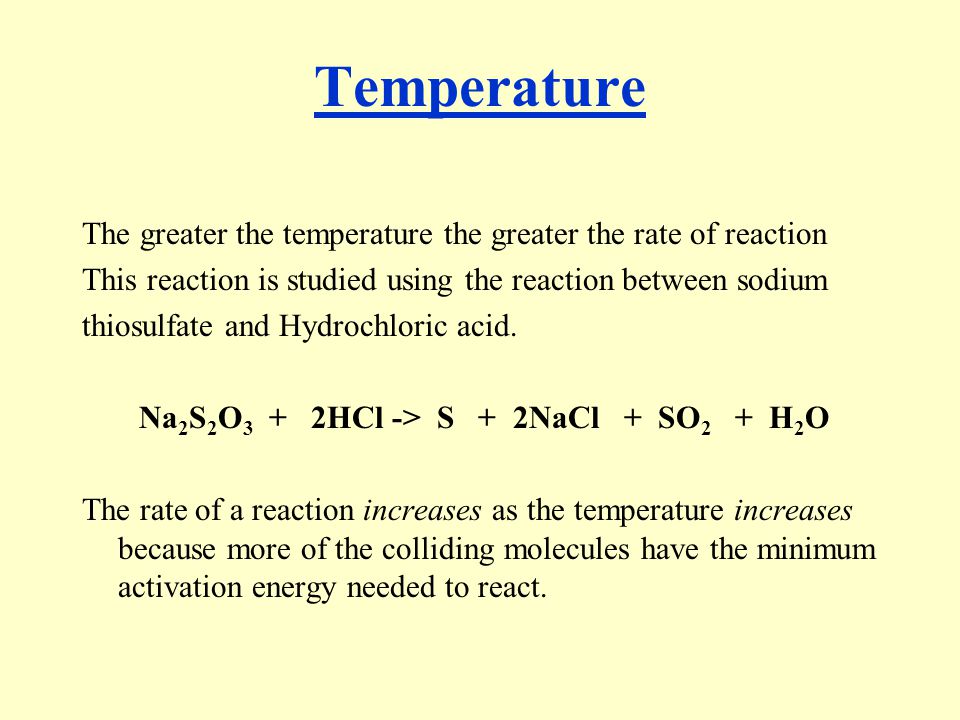
Investigating How the Concentration of Sodium Thiosulfate Within a Solution of Hydrochloric Acid Impacts on the Reaction Rate | Chemistry - Year 11 QCE | Thinkswap
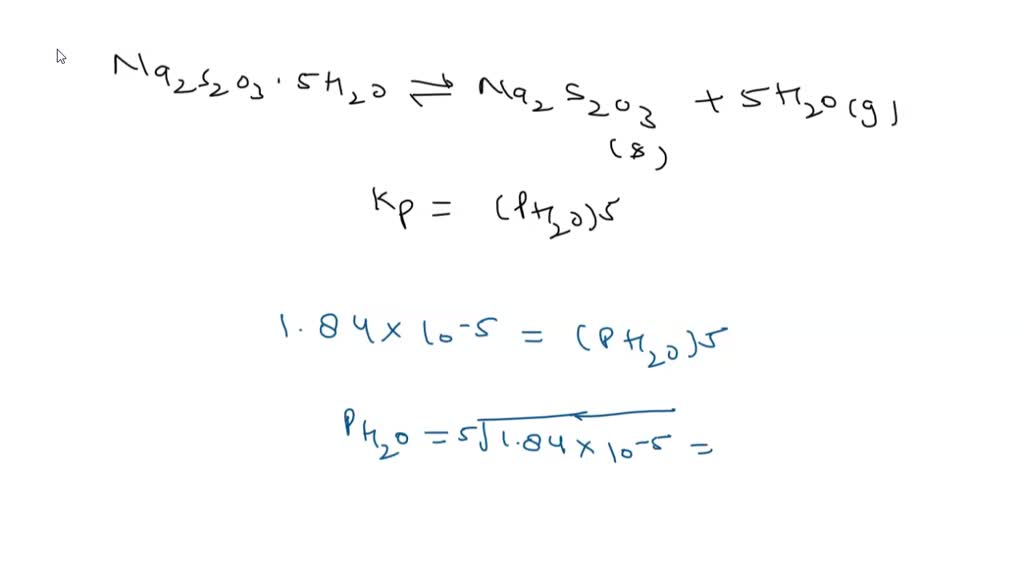
SOLVED: Sodium thiosulfate pentahydrate (FM 248.18 g ∙ mol-1) loses water when it is heated in an oven: Na2S2O3∙5H2O(s) ⇌ Na2S2O3(s) + 5H2O(g). ΔH° and ΔS° for this reaction at 25°C are
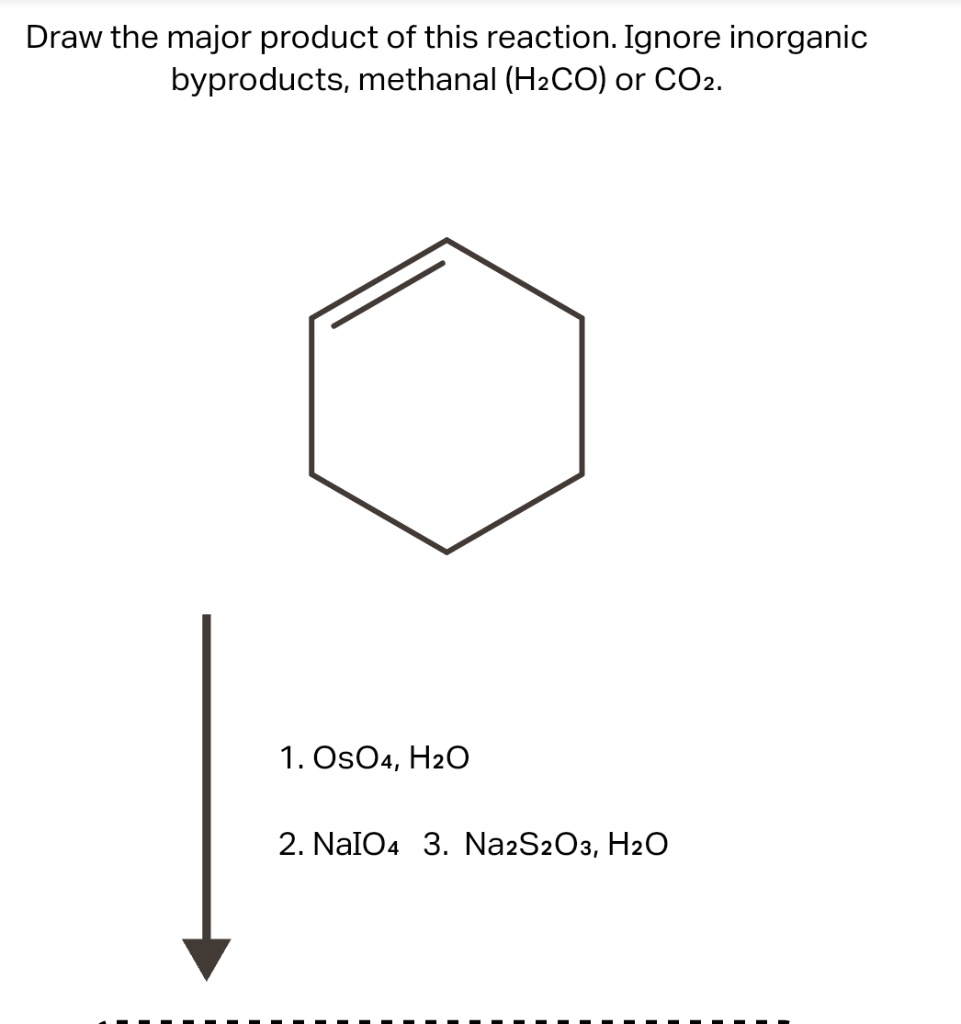
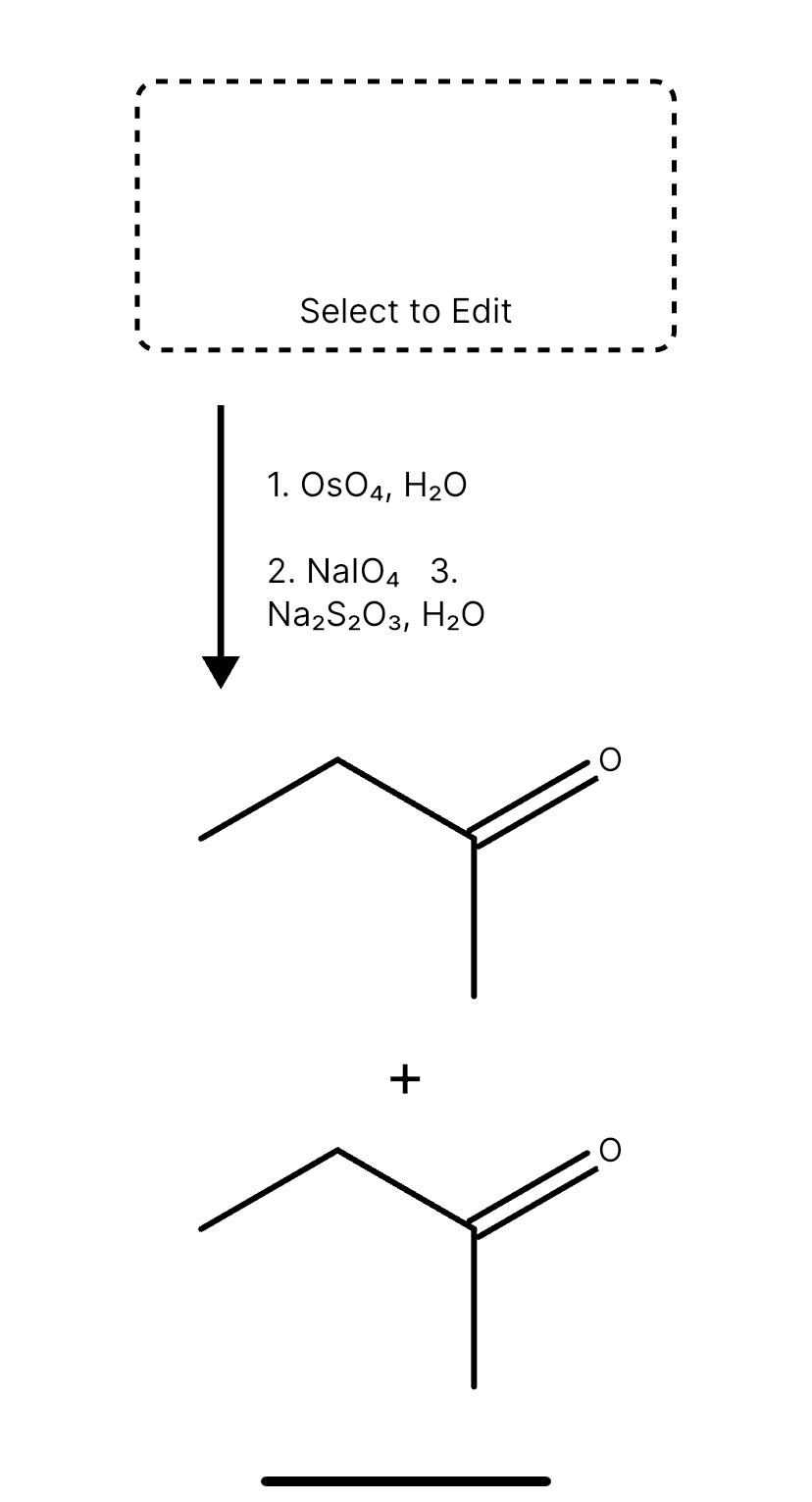
SOLVED: Draw the major product of this reaction. Ignore inorganic byproducts, methanal (H2CO) or CO2. 1.OsO4,H2O 2.NaIO4 3.Na2S2O3,H2O

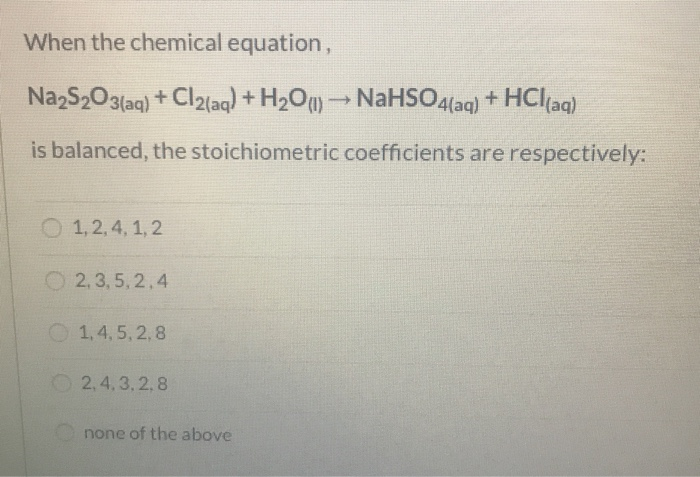
Aqueous solution of Na2S2O3 on reaction with Cl2 gives: NO 2 S 03 +12 (1) Na2S406 (2) NaHSO4 (3) NaCl (4) NaOH
Na2S2O3 has the molar mass M. In the reaction Na2S2O3 + H2O + Cl2 → Na2SO4 + 2HCl + S the equivalent weight of Na2S2O3 is

CAS 10102-17-7 Na2s2o3.5 H2O الصوديوم ثيوسولفات بينتاهدرات 99% - الصين ثيوسولفات الصوديوم، درجة ثيوسافات كبريتات الصناعية، ثيوديوم ثيوسولفات الصوديوم، بنتاهيرات، ثيوسلفات الصوديوم 99%، ثيوسايت دي سوديوم بري، ثيوسايت بنتاهيدرات، سوديوم ثيوسولفات بودر،

333. The system Na2S2O3–Ag2S2O3–H2O at 25° - Journal of the Chemical Society (Resumed) (RSC Publishing)
![One-Pot Synthesis of 2-R-Naphtho[2,3-b]thiophene-4,9-diones via Cyclization of 2-(R-Ethynyl)-1,4-naphthoquinones with Na2S2O3 | The Journal of Organic Chemistry One-Pot Synthesis of 2-R-Naphtho[2,3-b]thiophene-4,9-diones via Cyclization of 2-(R-Ethynyl)-1,4-naphthoquinones with Na2S2O3 | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.1c00852/asset/images/large/jo1c00852_0002.jpeg)
One-Pot Synthesis of 2-R-Naphtho[2,3-b]thiophene-4,9-diones via Cyclization of 2-(R-Ethynyl)-1,4-naphthoquinones with Na2S2O3 | The Journal of Organic Chemistry

The Rate of Reaction Between Sodium Thiosulphate and Hydrochloric Acid | PDF | Reaction Rate | Chemical Reactions

Помогите по химии. Написать общее выражение закона действующих масс для реакции : Na2S2O3+H2SO4 - Школьные Знания.com