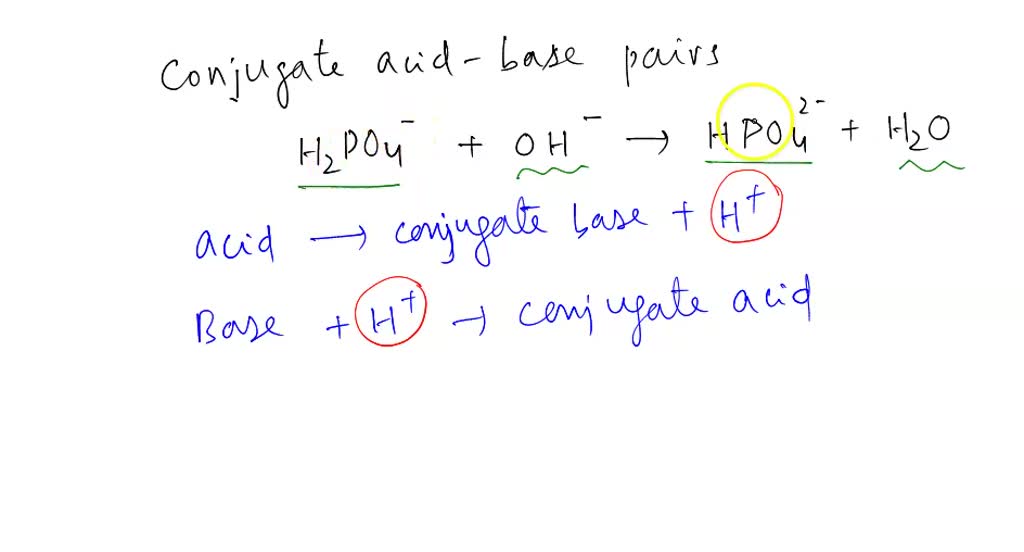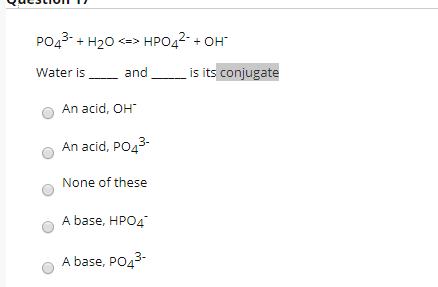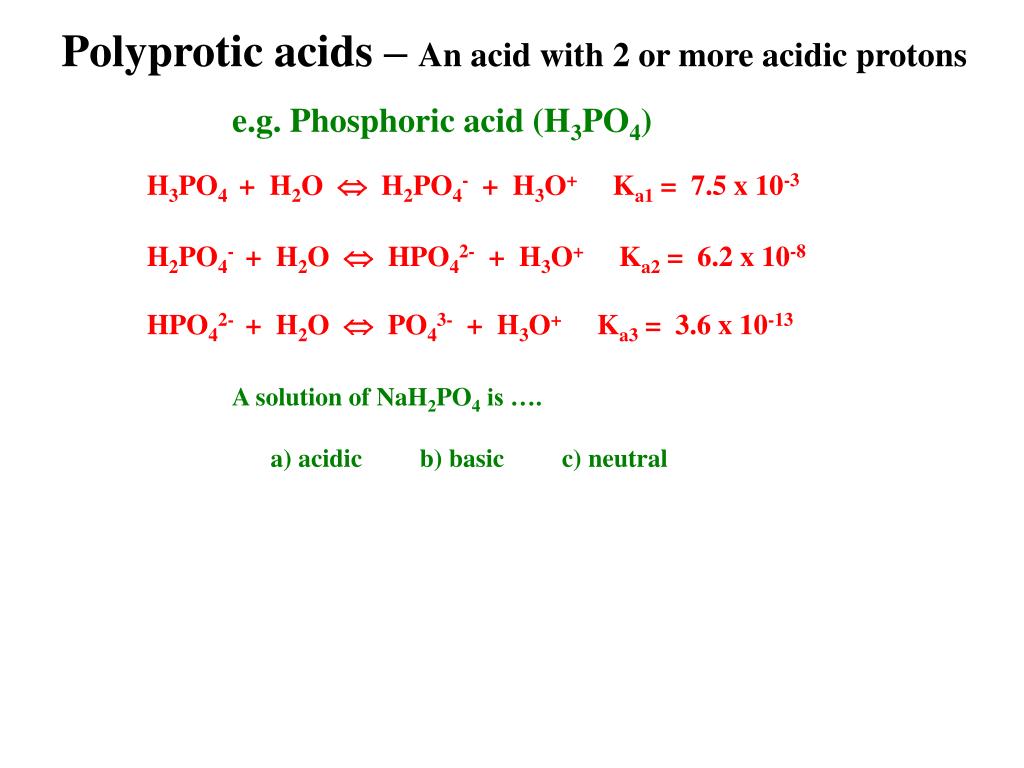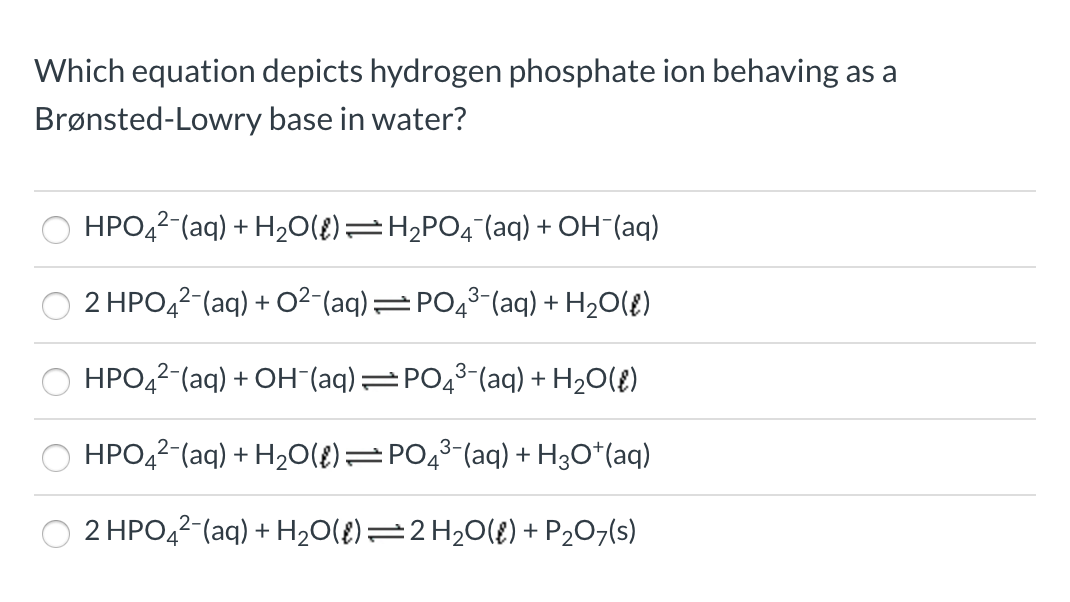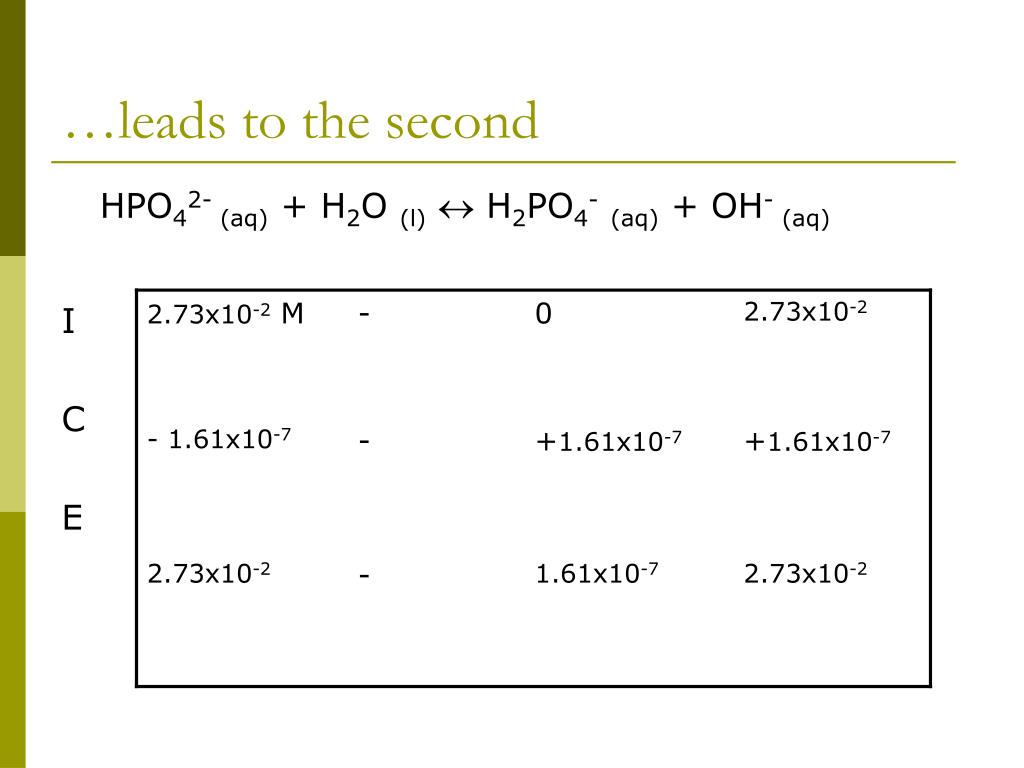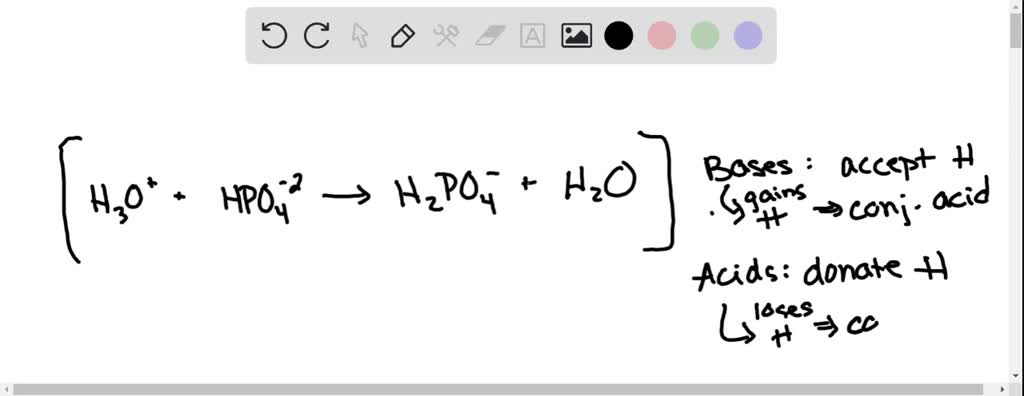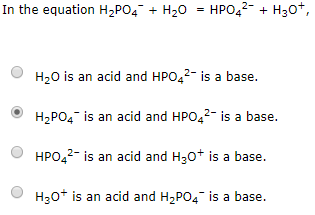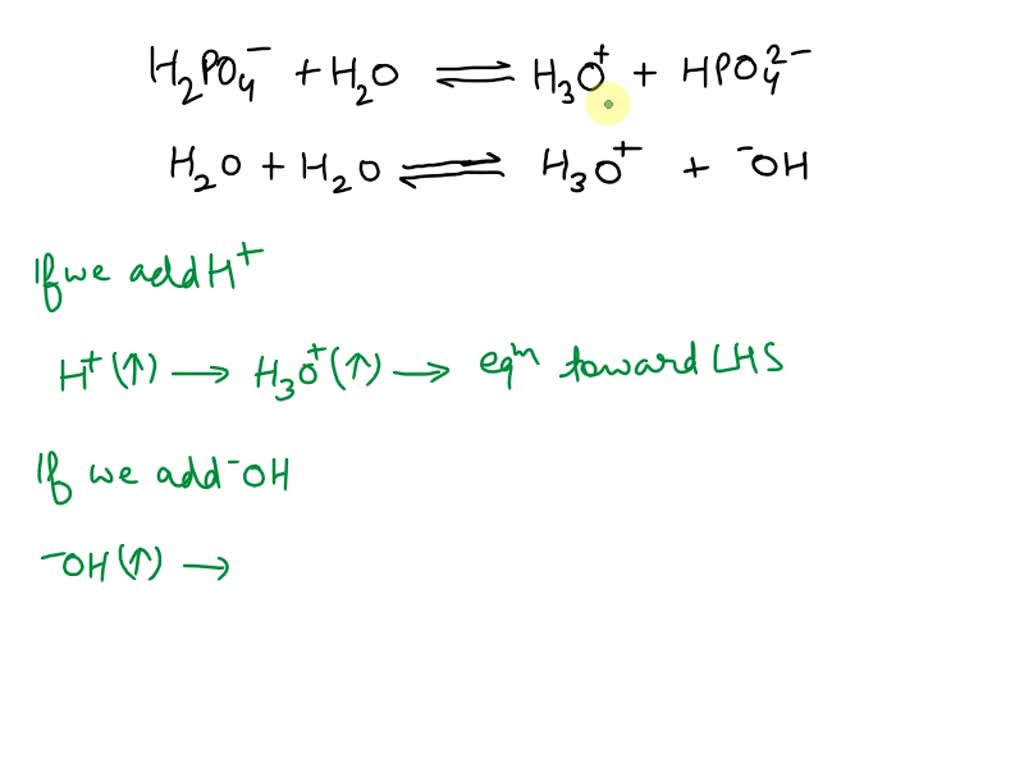
SOLVED: Relevant Equation H2PO4- + H2O <========> H3O+ + HPO4-2 Using Le Chatelier's Principle, explain why the phosphate buffer should have gone through a smaller change pH change compared to distilled water.
Synthesis and structural characterisation of solid titanium(IV) phosphate materials by means of X-ray absorption and NMR spectro

Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol Using Ag@α-Ti(HPO4)2· H2O: Experimental and Computational Studies | Industrial & Engineering Chemistry Research

Catalytic conversions of isocyanate to urea and glucose to levulinate esters over mesoporous α-Ti(HPO4)2·H2O in green media - X-MOL

PDF) Thermal decomposition kinetics and reversible hydration study of the Li2Zn(HPO4)2�H2O | Chanaiporn Danvirutai - Academia.edu

Single-crystal neutron diffraction and Mössbauer spectroscopic study of hureaulite, (Mn,Fe)5(PO4)2 (HPO4)2 (H2O)4 - European Journal of Mineralogy Volume 28 Number 1 — Schweizerbart science publishers
CasNo.13772-29-7,Zirconium Phosphate Molecular Formula Zr(HPO4)2·H2O Change The Sewage Become Cleaning Water,(13772-29-7) Suppliers

Figure 5. XRD of Zr0.8Ti0.2(HPO4)2.H2O : α- Zirconium Titanium Phosphates - Fibrous Cerium Phosphate Composite Membranes and Their 1,10- Phenanthroline Cu(II) Pillared Materials : Science and Education Publishing
![Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.1c02685/asset/images/large/ic1c02685_0008.jpeg)
Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry

Nucleation & growth of α-Ti(HPO4)2·H2O single-crystal and its structure determination from X-ray single–crystal data - ScienceDirect

PDF) Neutron powder diffraction study of α-Ti(HPO4)2.H2O and α-Hf(HPO4)2.H2O; H-atom positions | Pilar Pertierra - Academia.edu