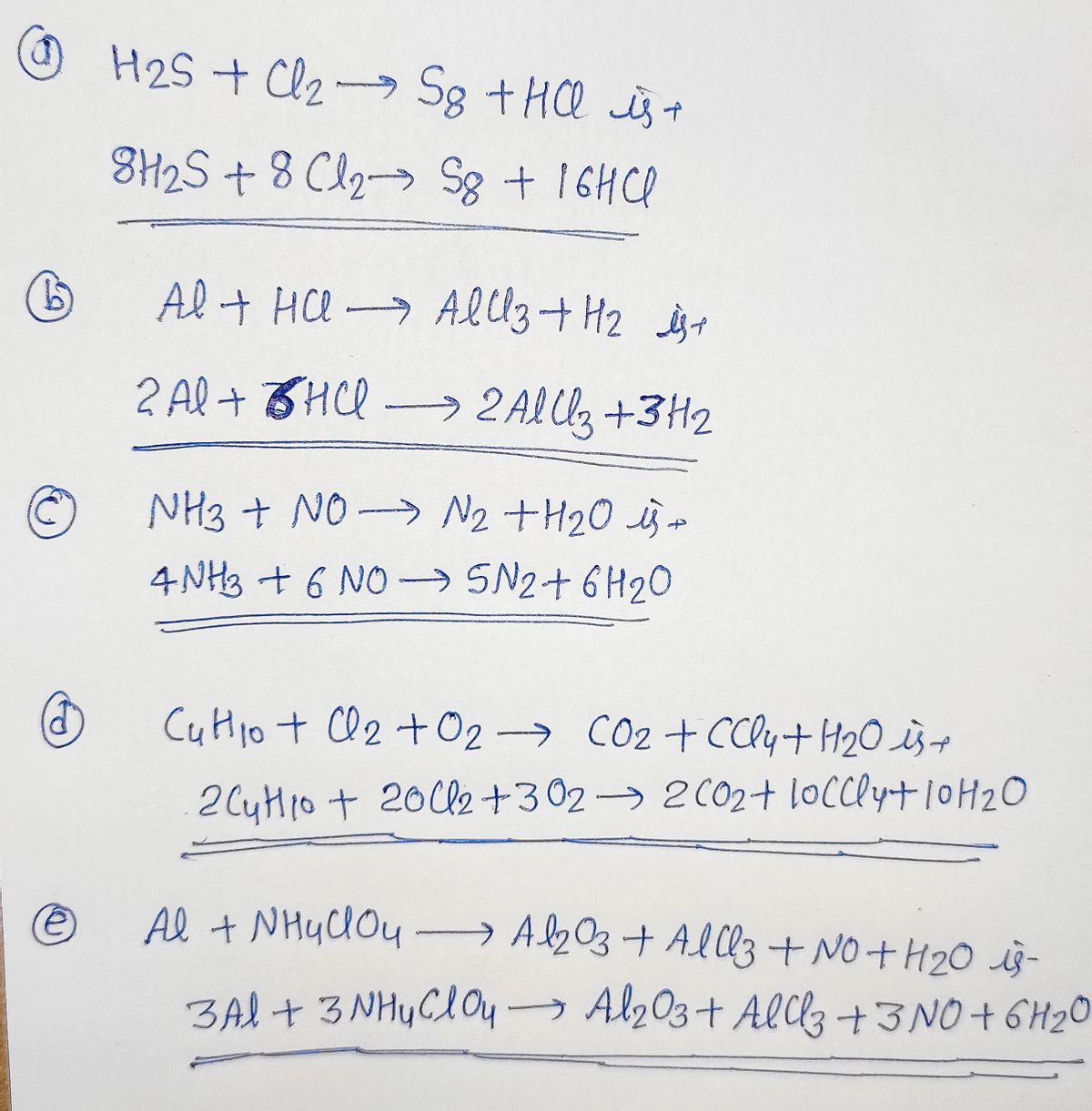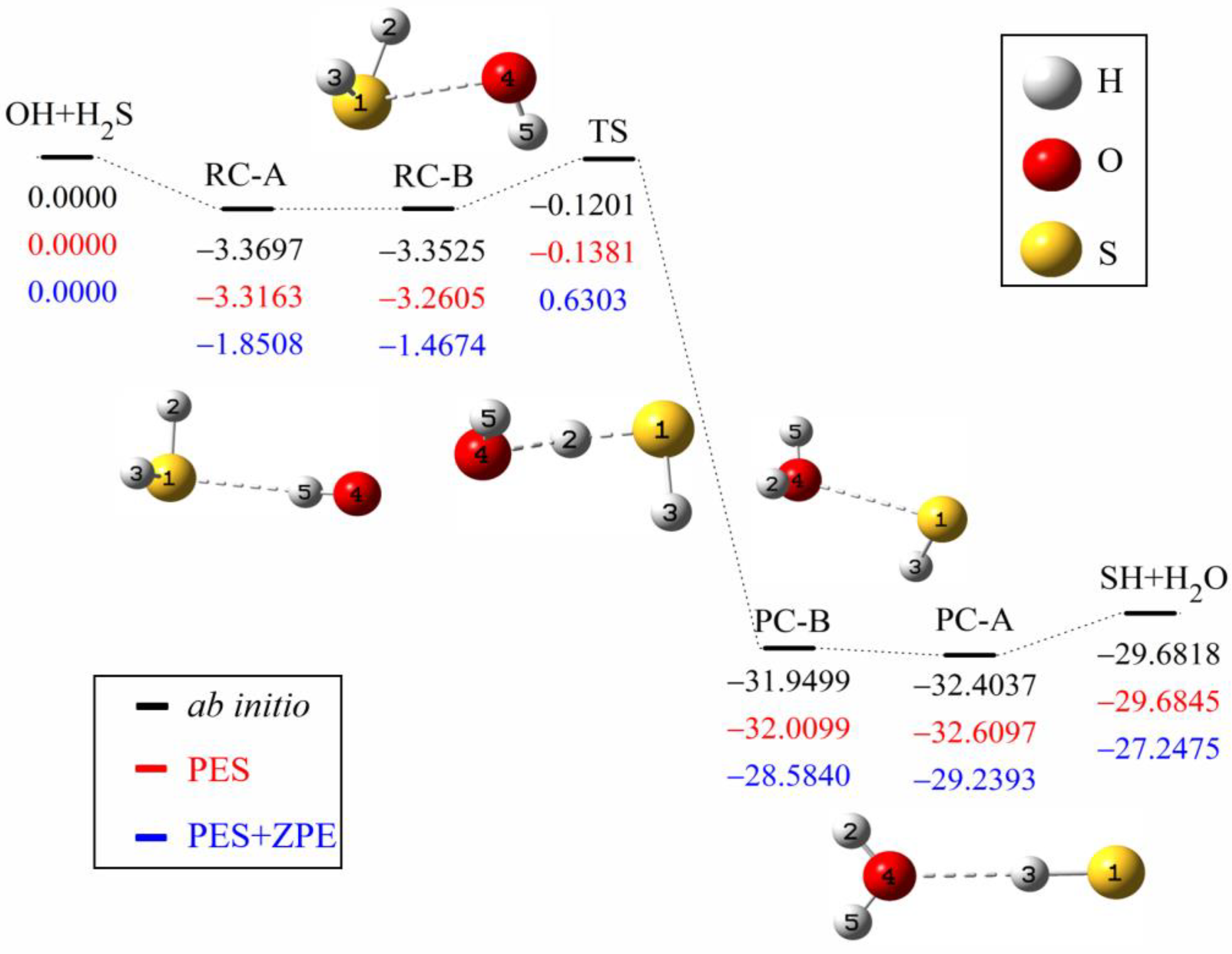
Symmetry | Free Full-Text | Quasi-Classical Trajectory Dynamics Study of the Reaction OH + H2S→H2O + SH and Its Isotopic Variants: Comparison with Experiment

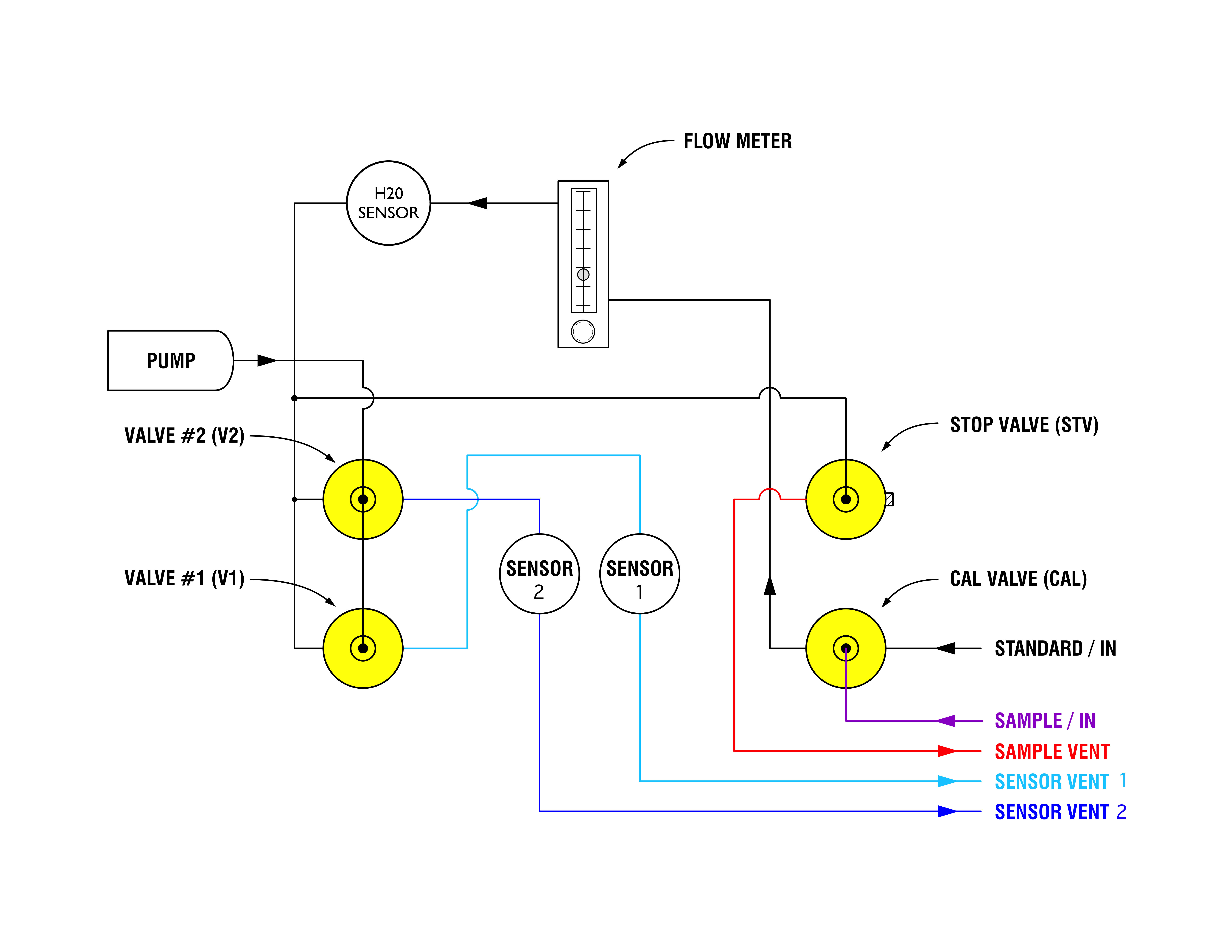
Hydrogen Sulfide, Corrosion and BOD5 in Innovyze InfoSewer – ICM SWMM & ICM InfoWorks, SWMM5 & SWMM5+

Hydrate Stability in the H2S–H2O system—Visual Observations and Measurements in a High-Pressure Optical Cell and Thermodynamic Models | Journal of Chemical & Engineering Data

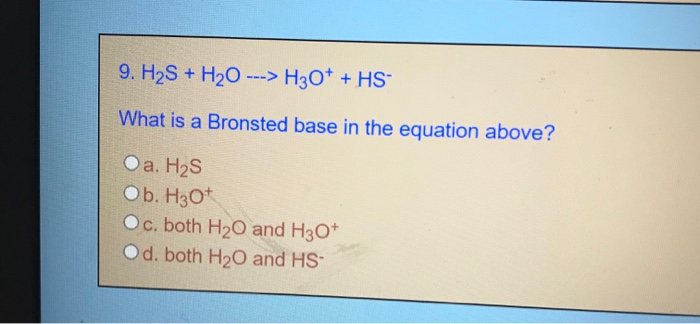
SOLVED: QUESTION 11 Is H2O or H2S more nucleophilic in protic solvent, and why? H2S is more nucleophilic because it is less solvated by the protic solvent: 0 H2O is more nucleophilic

How to Balance H2SO4 + HI = H2S + I2 + H2O We are back with another video wherein we help you balance the equation. It is a redox equation and for

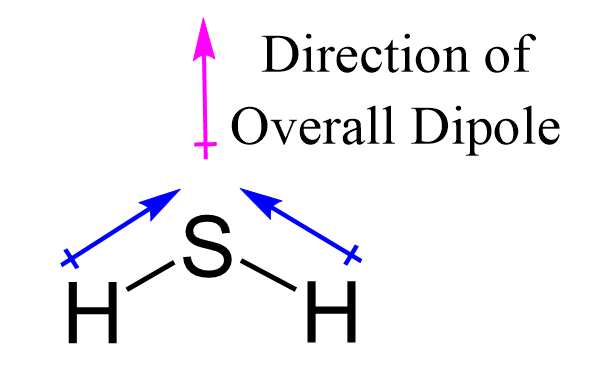
![Punjabi] H2S is a gas while H2O is liquid at room temperature·? Why ? Punjabi] H2S is a gas while H2O is liquid at room temperature·? Why ?](https://static.doubtnut.com/ss/web-overlay-thumb/10548186.webp)