Crystal structure of Cu(OH) 2 (a) and a proposed structure model of... | Download Scientific Diagram

Figure 2 from Simple Template-Free Solution Route for the Controlled Synthesis of Cu(OH)2 and CuO Nanostructures | Semantic Scholar

Fabrication and characterization of Cu(OH) 2 /CuO nanowires as a novel sensitivity enhancer of the luminol–H 2 O 2 chemiluminescence system: determina ... - RSC Advances (RSC Publishing) DOI:10.1039/C5RA21085B
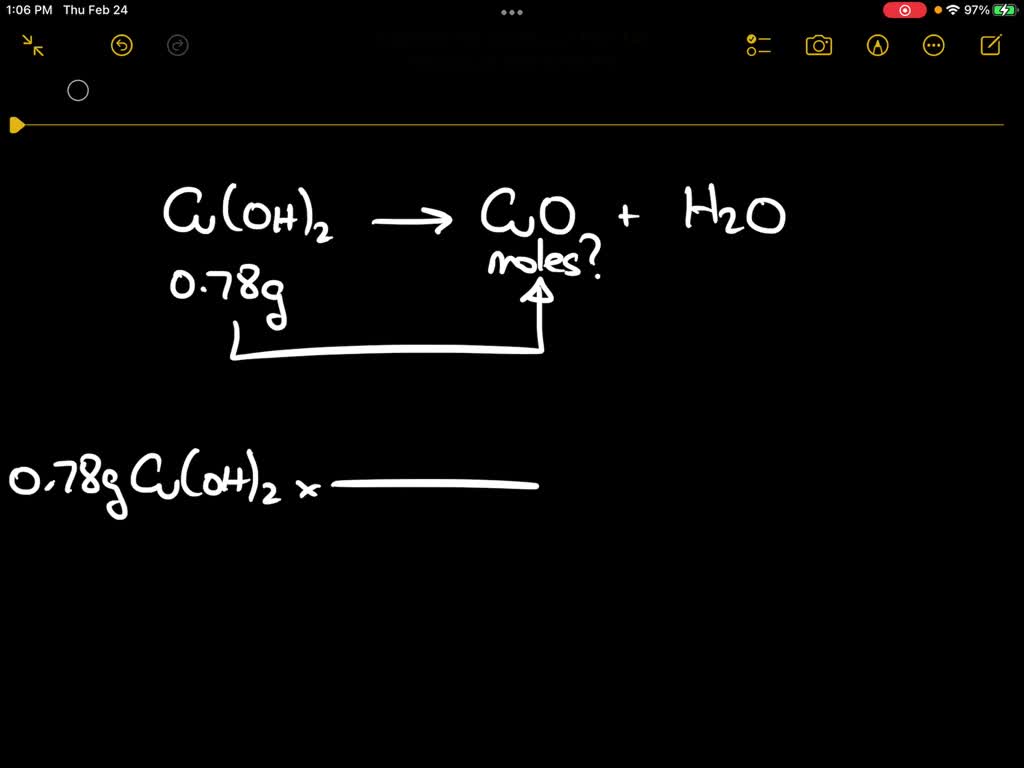
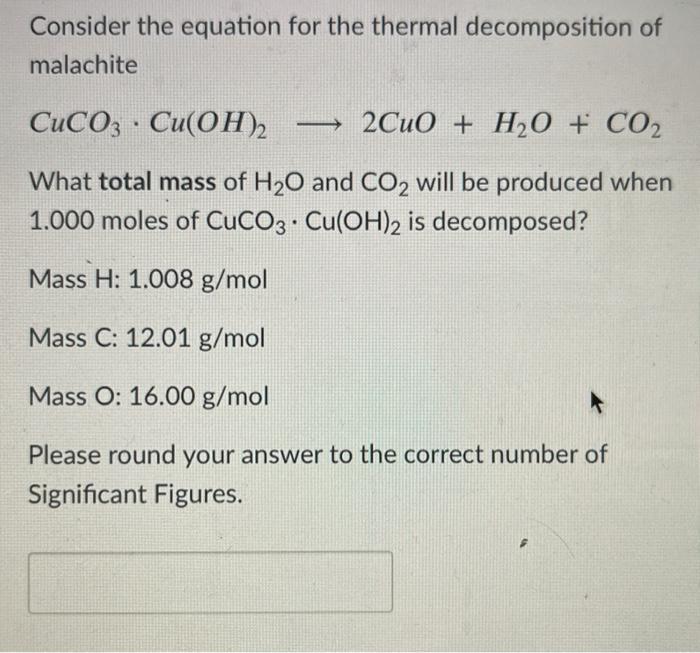
SOLVED: Upon heating Cu(OH)2 decomposes into CuO and H2O as shown below: Cu(OH)2 â†' CuO + H2O. If 0.78 g of Cu(OH)2 was decomposed, calculate the moles of CuO produced. Use 29Cu63.5,
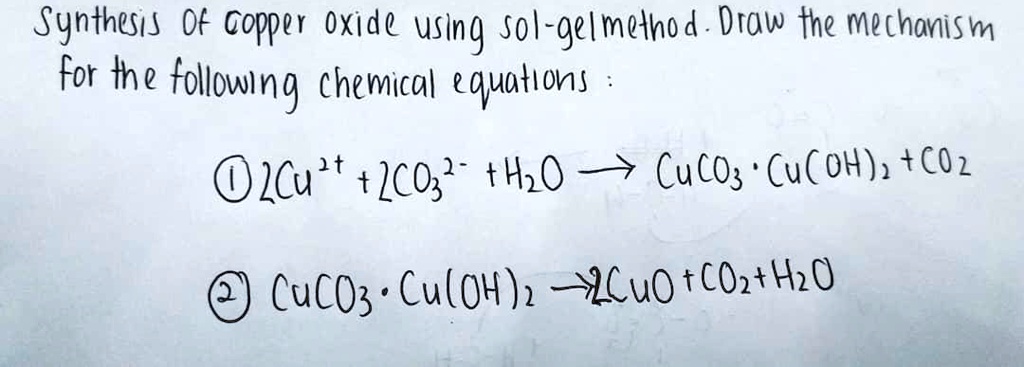
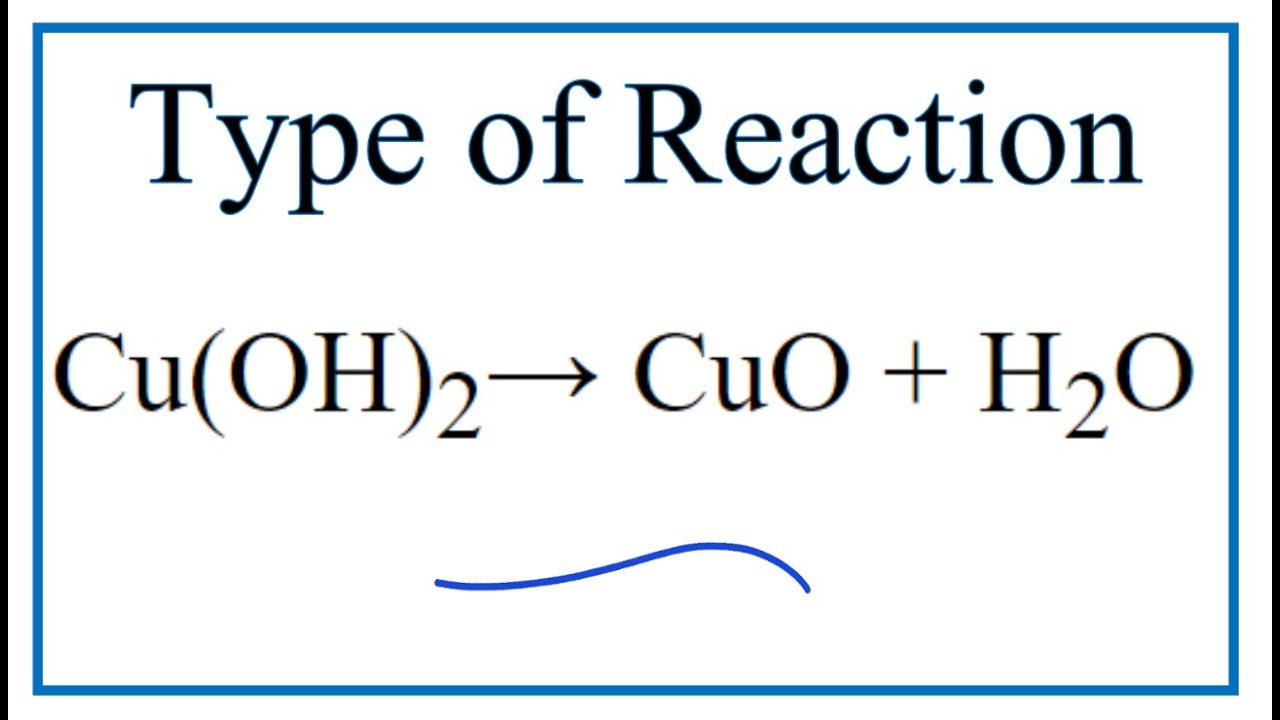
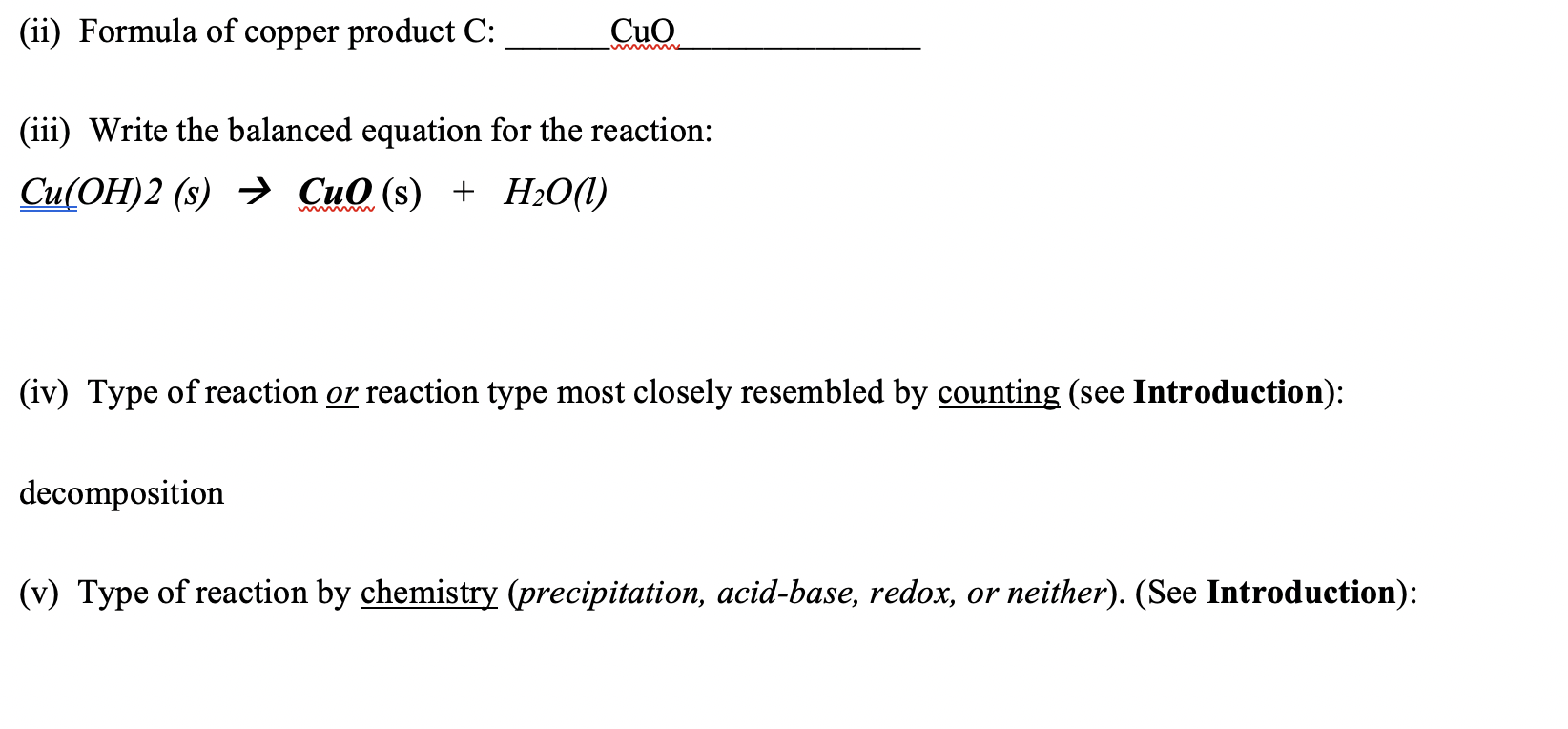
SOLVED: Synthesis of Copper Oxide using Sol-gel method. Draw the mechanism for the following chemical equation: Cu(OH)2 + 2CO3 -> CuCO3 + H2O. CuCO3 -> Cu(OH)2 + CO2. CuCO3 -> CuO + CO2 + H2O.

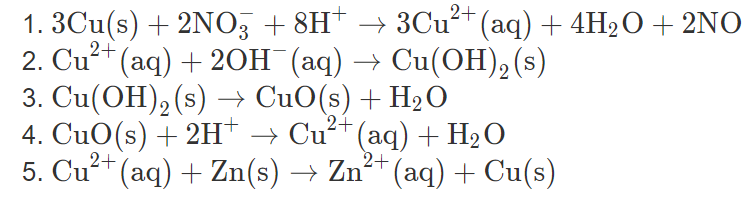

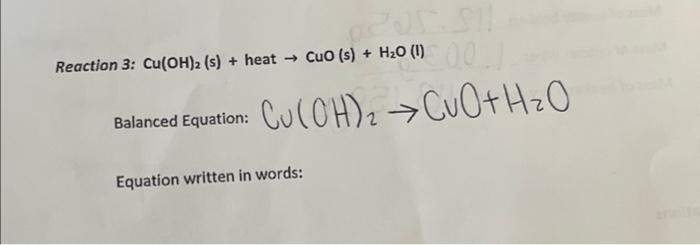





![Cu(OH2)6]2+ - Copper(II) hydroxide Cu(OH2)6]2+ - Copper(II) hydroxide](https://www.chemtube3d.com/images/gallery/inorganicsjpgs/cuoh262_.jpg)