Synthesis, Crystal Structure and Antitumor Activity of a Ca(II) Coordination Polymer Based on 4-Acetylphenoxyacetate Ligands((1)),Chinese Journal of Structural Chemistry - X-MOL

SOLVED: How many mL of 0.727 M HCl are needed to dissolve 7.78 g of CaCO3? HCl(aq) + CaCO3(s) -> CaCl2(aq) + H2O(l) + CO2(g)
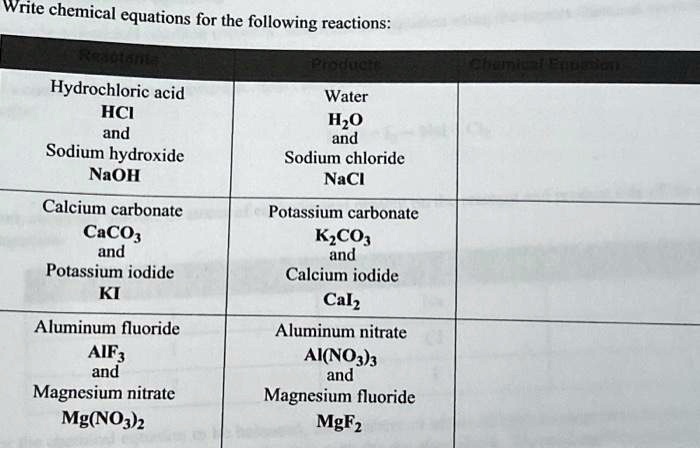
SOLVED: Write chemical equations for the following reactions: Hydrochloric acid HCl and Sodium hydroxide NaOH Calcium carbonate CaCO3 and Potassium iodide KI Water H2O Sodium chloride NaCl Potassium carbonate K2CO3 and Calcium

SOLVED: To study the impact of acid rain on sea organisms, oyster shells were exposed to an acidic solution HX. It is known that oyster shells contain calcium carbonate. Which of the

Solubilities in the system Np(V)–H–Na–OH–ClO4–CO2/CO3–H2O as a function... | Download Scientific Diagram
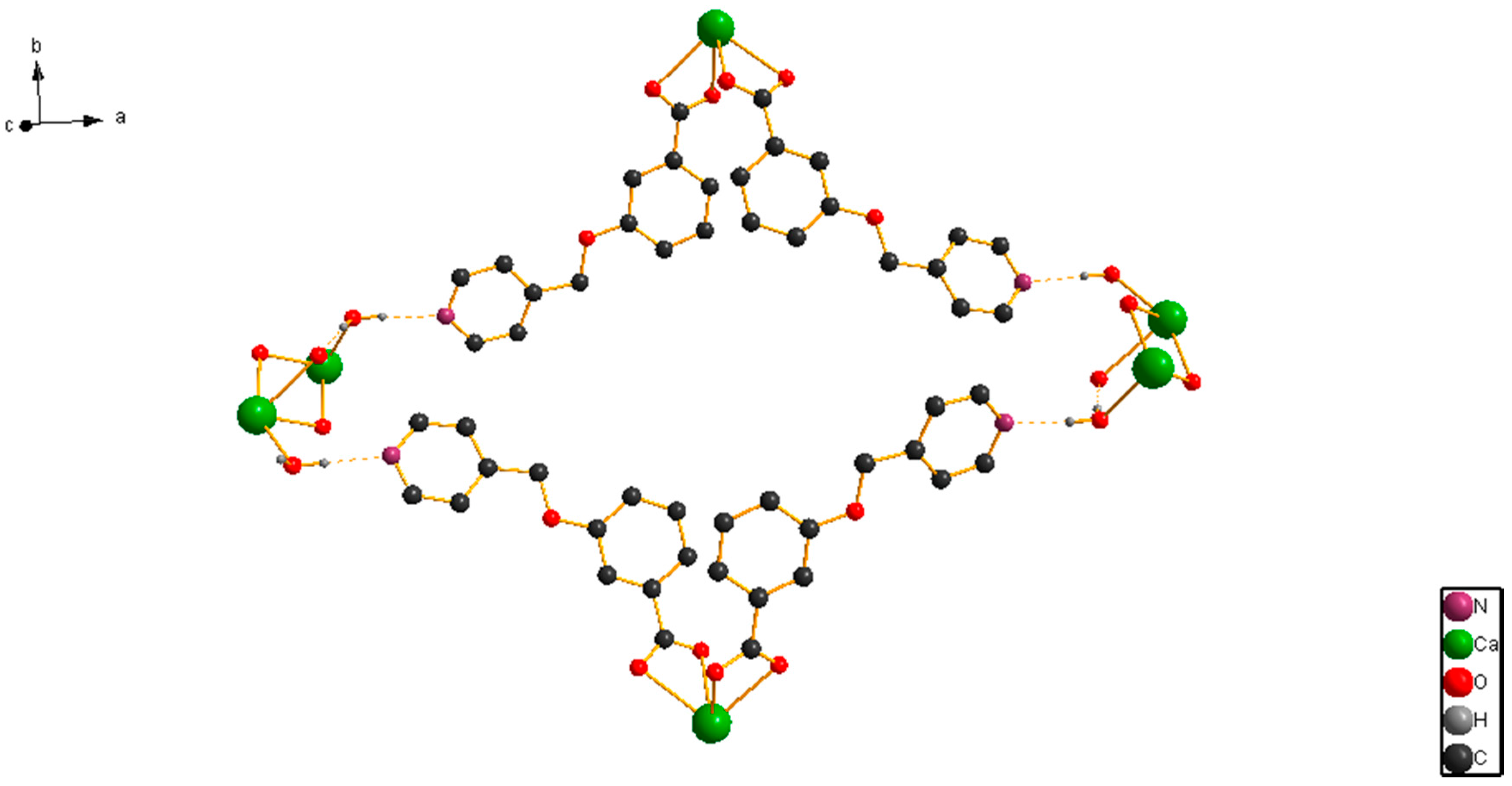
Crystals | Free Full-Text | Synthesis, Crystal Structure and Catalytic Activity of a 1D Chained Ca(II) Coordination Polymer with 3,5-Bis(4-pyridylmethoxy)benzoate Ligand
![PDF] Simultaneous structural and dielectric measurement of ammonia storage materials | Semantic Scholar PDF] Simultaneous structural and dielectric measurement of ammonia storage materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/48c866f029c1b60aabfab419bdc51dd3189fccef/73-Table4.2-1.png)
PDF] Simultaneous structural and dielectric measurement of ammonia storage materials | Semantic Scholar

Crystals | Free Full-Text | Synthesis, Crystal Structure and Catalytic Activity of a 1D Chained Ca(II) Coordination Polymer with 3,5-Bis(4-pyridylmethoxy)benzoate Ligand

Figure 1 from A Ca(II) Coordination Polymer of 2-Carboxybenzaldehyde: Synthesis, Crystal Structure, and Catalytic Activity in Oxidation of Benzyl Alcohol | Semantic Scholar

Phase Equilibrium in the Ternary System K2O–Al2O3–H2O at 323.15, 333.15, 343.15, and 353.15 K | Journal of Chemical & Engineering Data

Comparison of the Microsolvation of CaX2 (X = F, Cl, Br, I) in Water: Size-Selected Anion Photoelectron Spectroscopy and Theoretical Calculations | The Journal of Physical Chemistry A