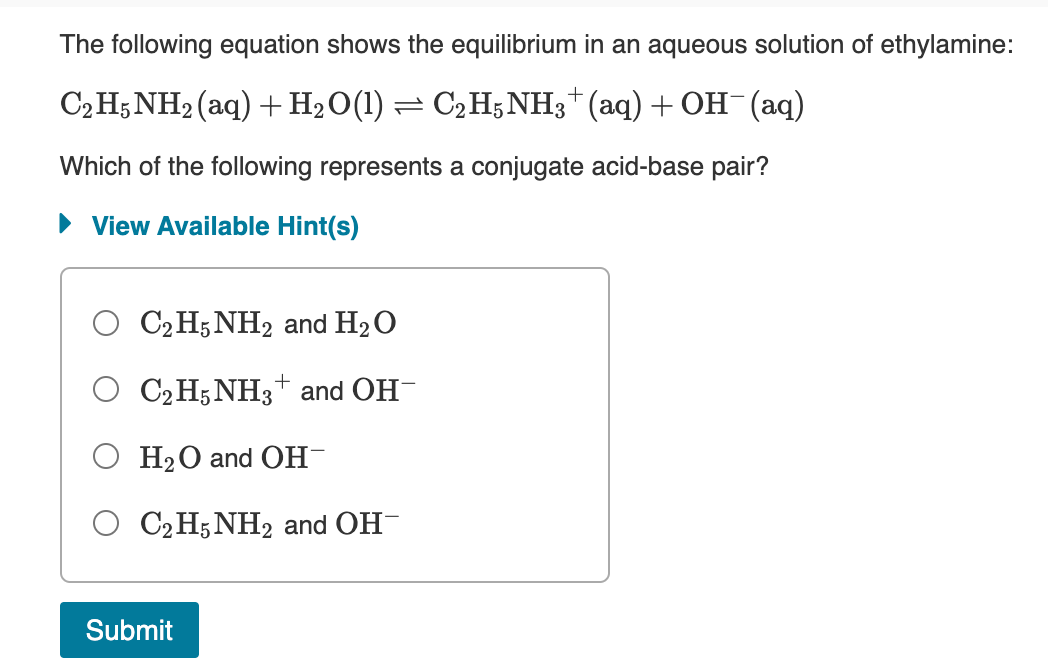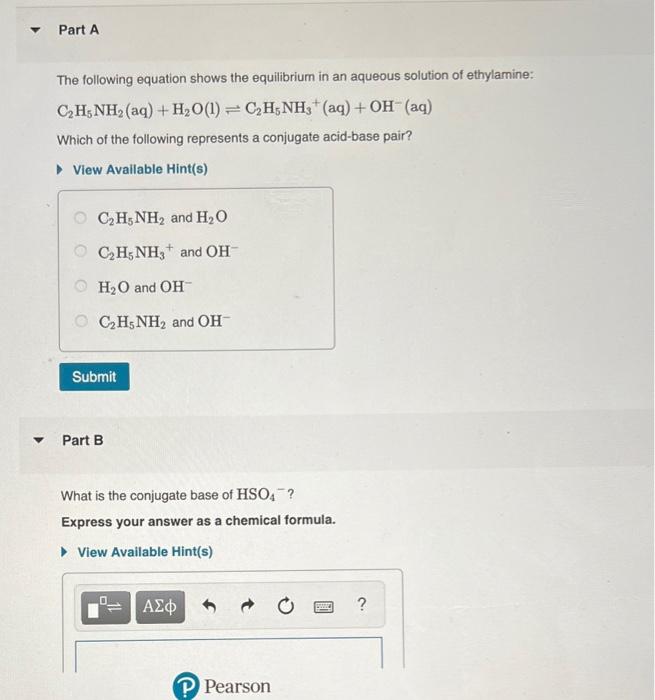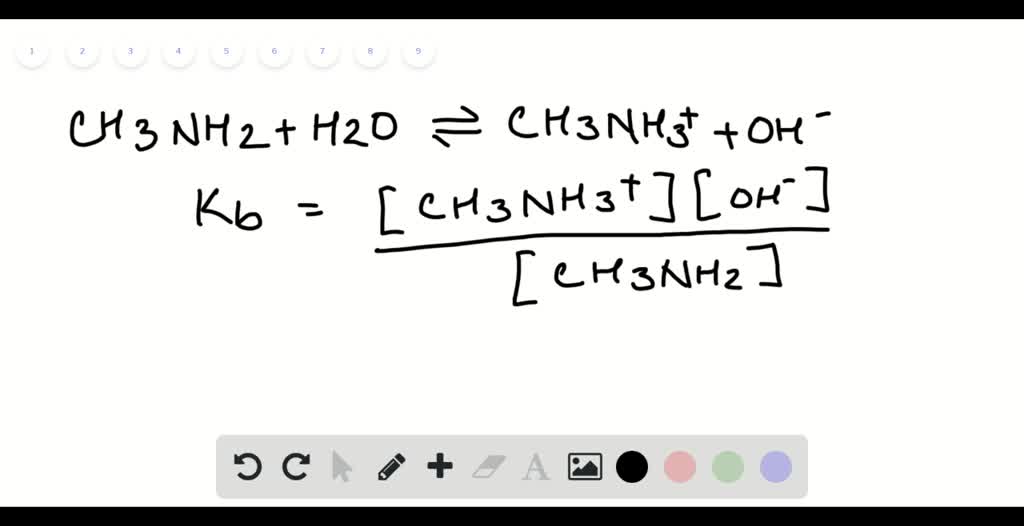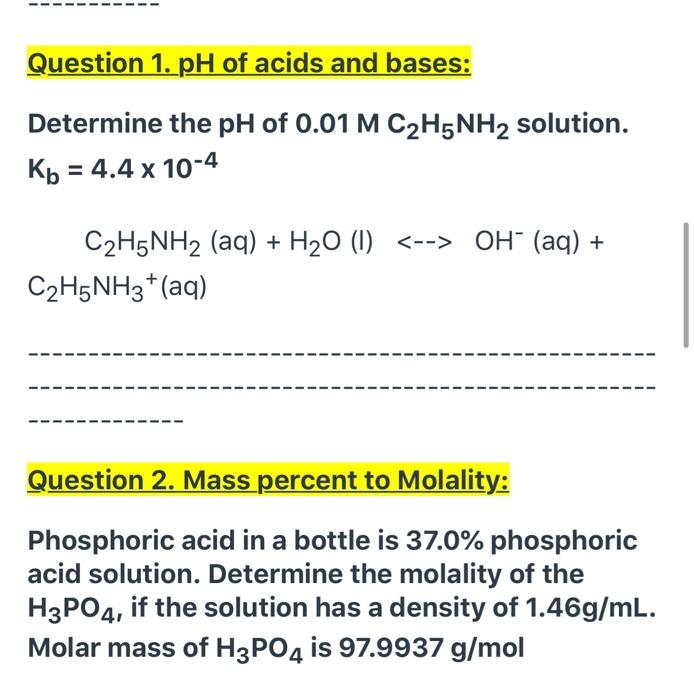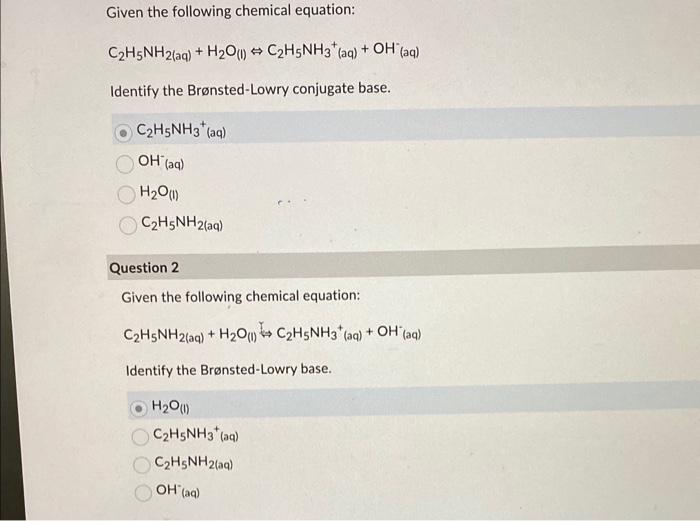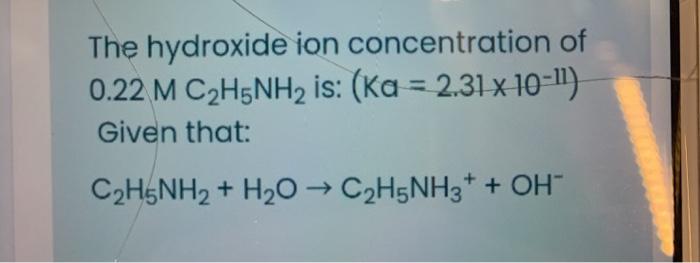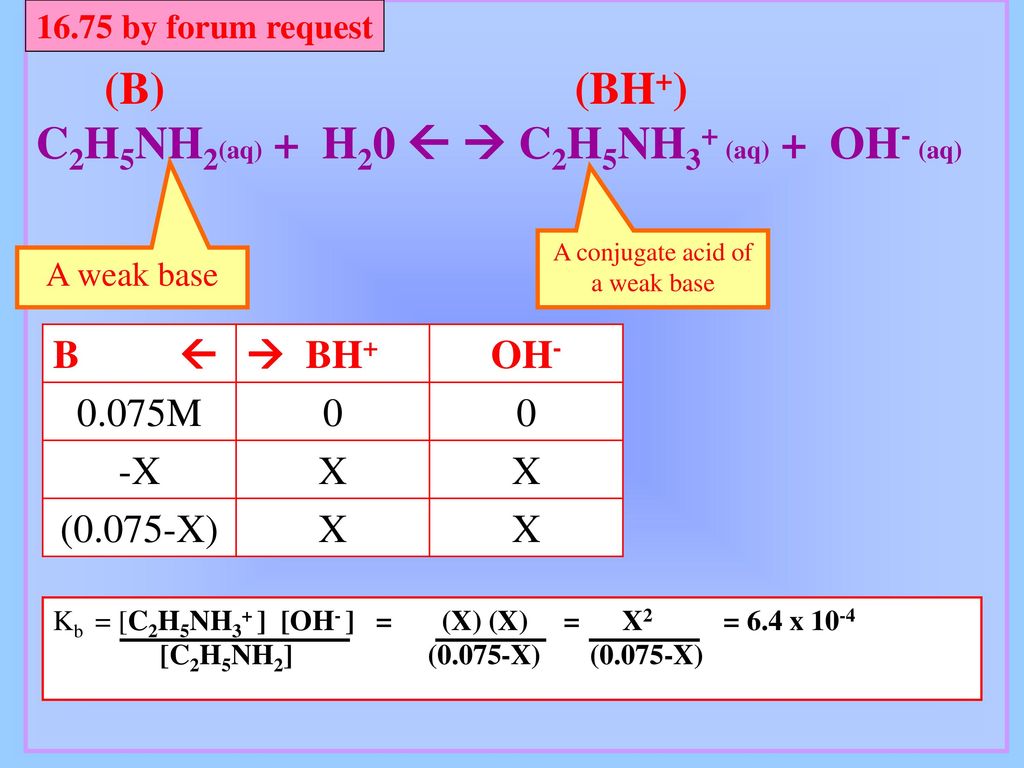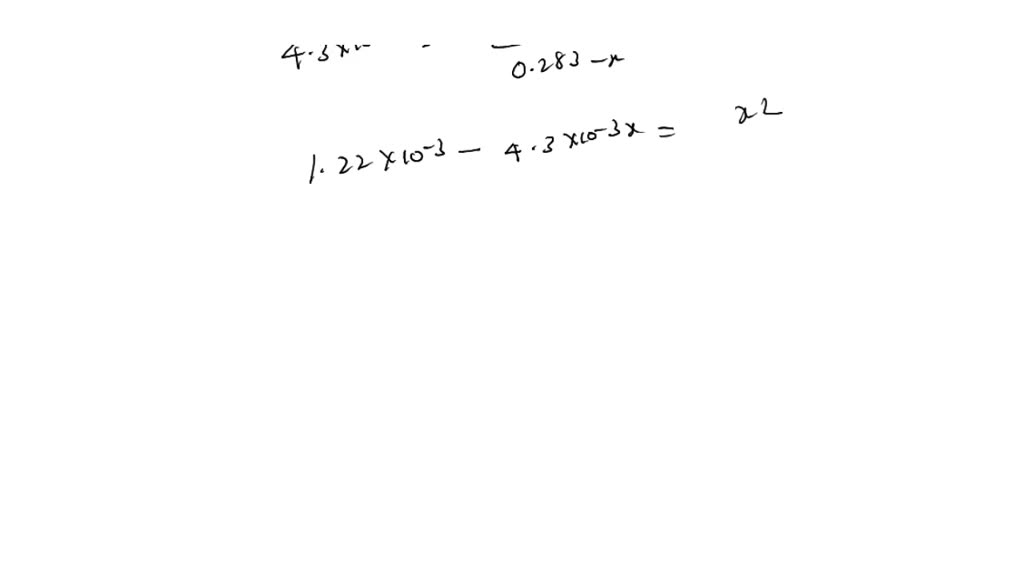
SOLVED: C2H5NH2 + H2O <–> C2H5NH3 +OH- A 0.283 M solution of C2H3NH2 was created. Calculate the equilibrium concentrations of all species, and the pH of the solution.
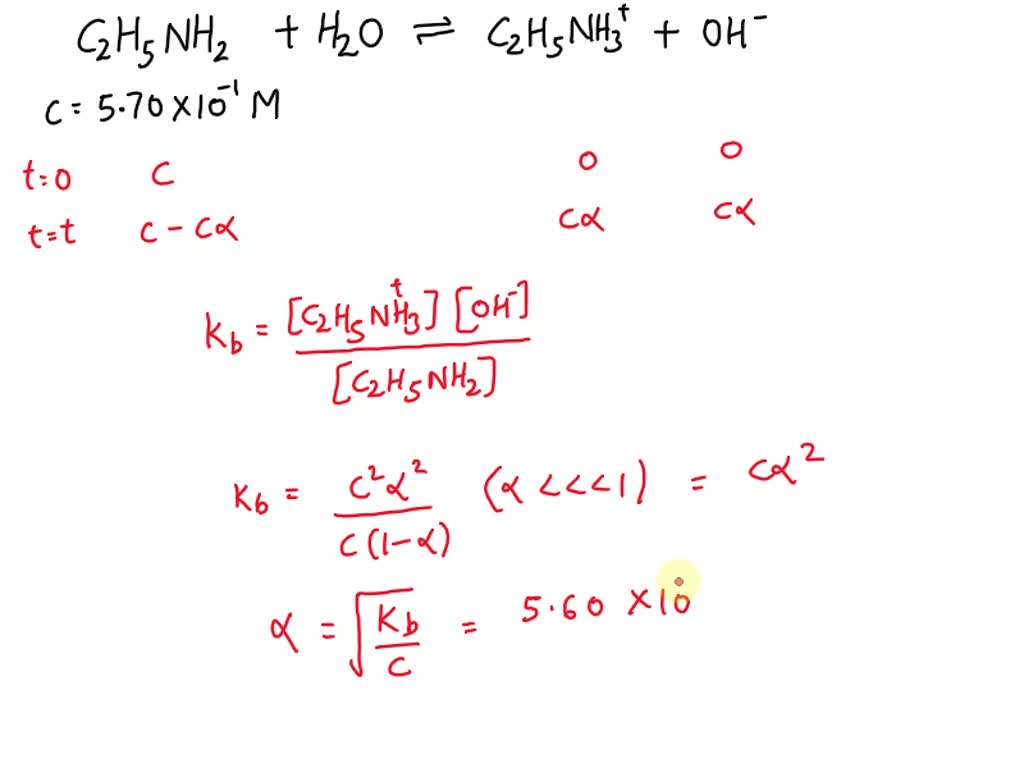
SOLVED: Calculate the pH of a 5.70×10^-1 M aqueous solution of ethylamine hydrochloride (C2H5NH3Cl). (For ethylamine, C2H5NH2, Kb = 5.60×10^-4.)

Max. Marks : 70 an 4. Complete the following reaction. CO → A -KI NH KOH (alc.) CHI NH H2O CO - HO C + C2H5NH2 Ethylamine
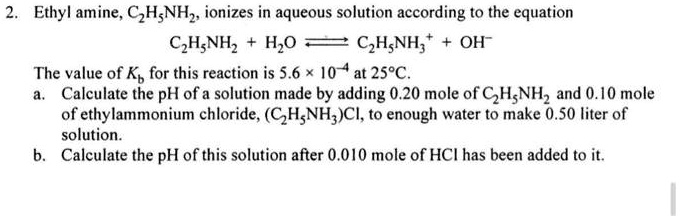
SOLVED: Ethylamine, C2H5NH2, ionizes in aqueous solution according to the equation C2H5NH2 + H2O ⇌ C2H5NH3+ + OH-. The value of Kb for this reaction is 5.6 x 10^4 at 25°C. Calculate
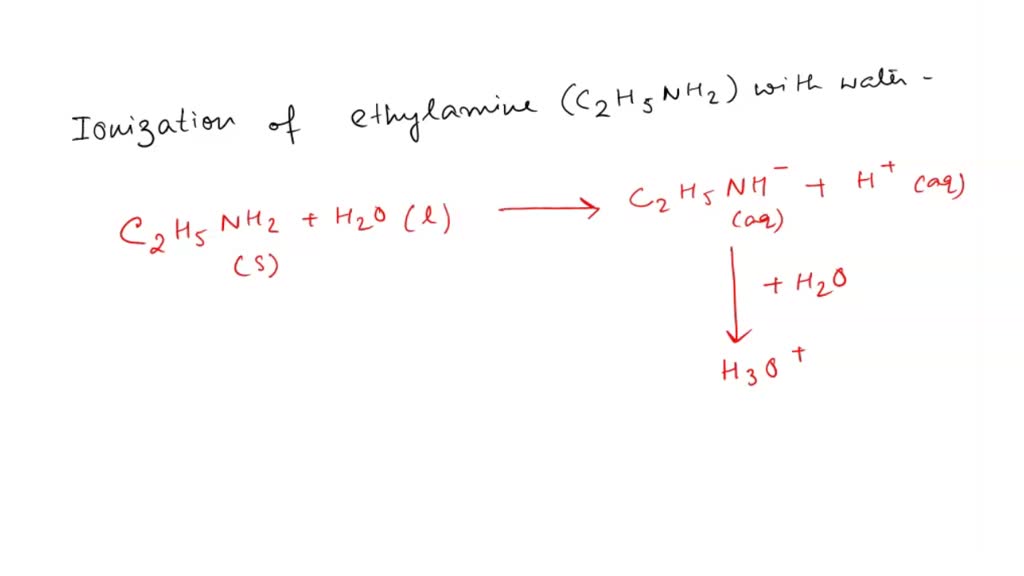
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water
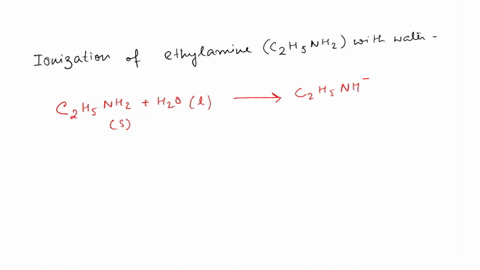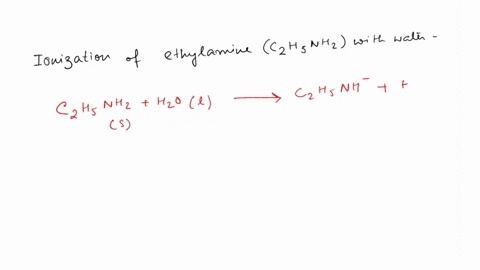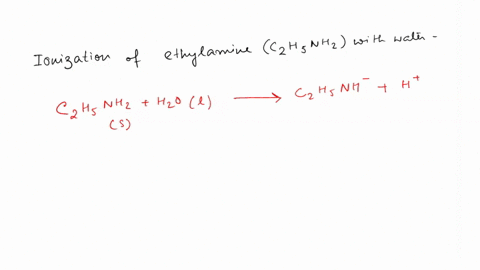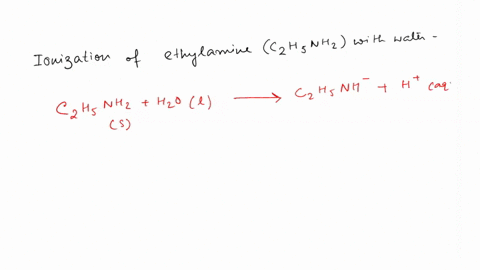
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water