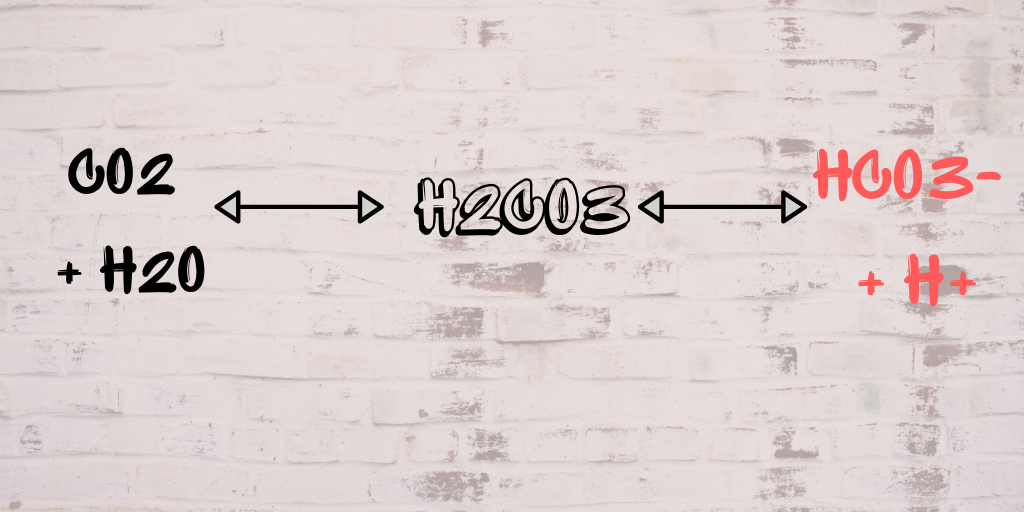
Write fully balanced equations for the following : (a) CO2 + H2O → ............ - Sarthaks eConnect | Largest Online Education Community

Phase equilibria in the H2O–CO2 system between 250–330 K and 0–1.7 GPa: Stability of the CO2 hydrates and H2O-ice VI at CO2 saturation - ScienceDirect

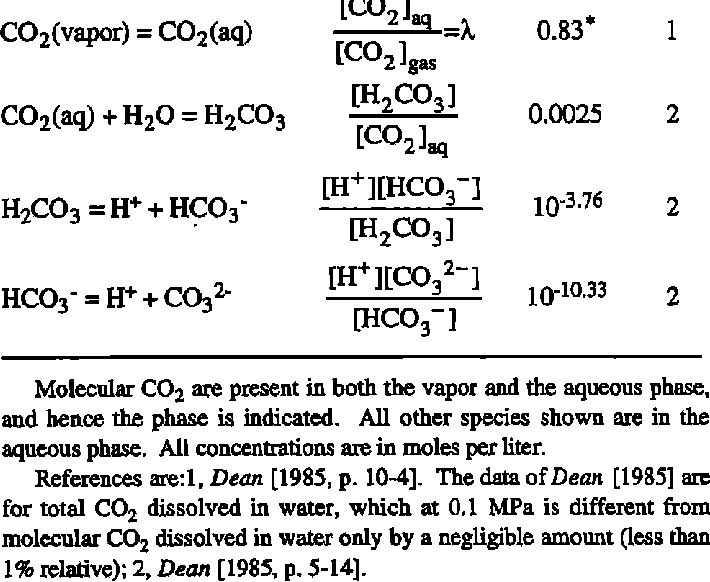
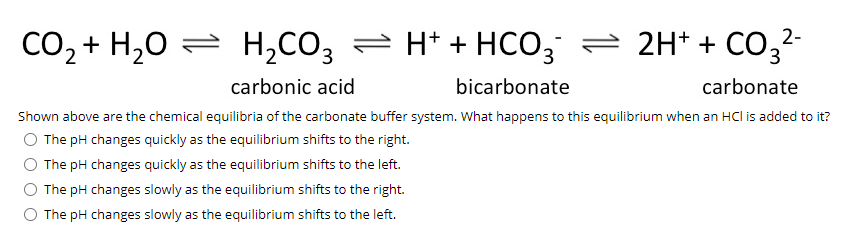
Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram

Data-Driven Many-Body Models for Molecular Fluids: CO2/H2O Mixtures as a Case Study | Journal of Chemical Theory and Computation
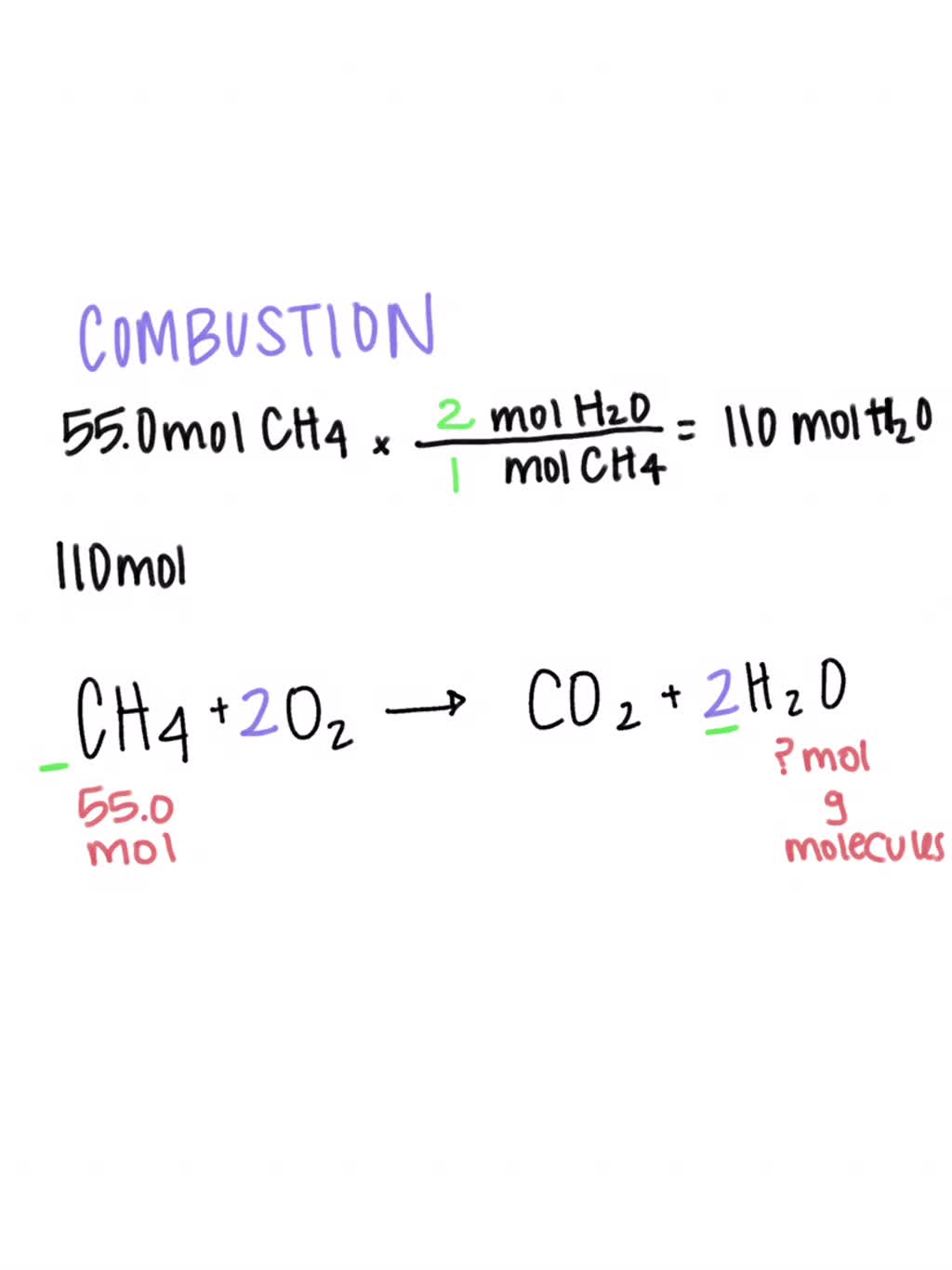
SOLVED: CH4 + O2 ——————- CO2 + H2O What type of reaction does this equation represent? Write and balance the equation. Assume that there are 55.0 moles of CH4. How many moles

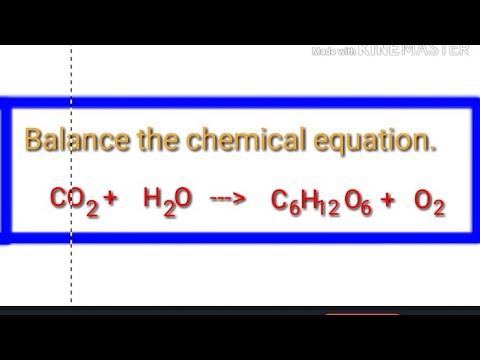
CO2+H2O=C6H12O6+O2+H2O balance the chemical equation @mydocumentary838. co2+ h2o=c6h12o6+o2+h2o - YouTube

Write fully balanced equations for the following:(a) CO2 + H2O(b) Ca(OH)2+ CO2(c) SO2 + H2O(d) P205 + H2O(e) - Brainly.in