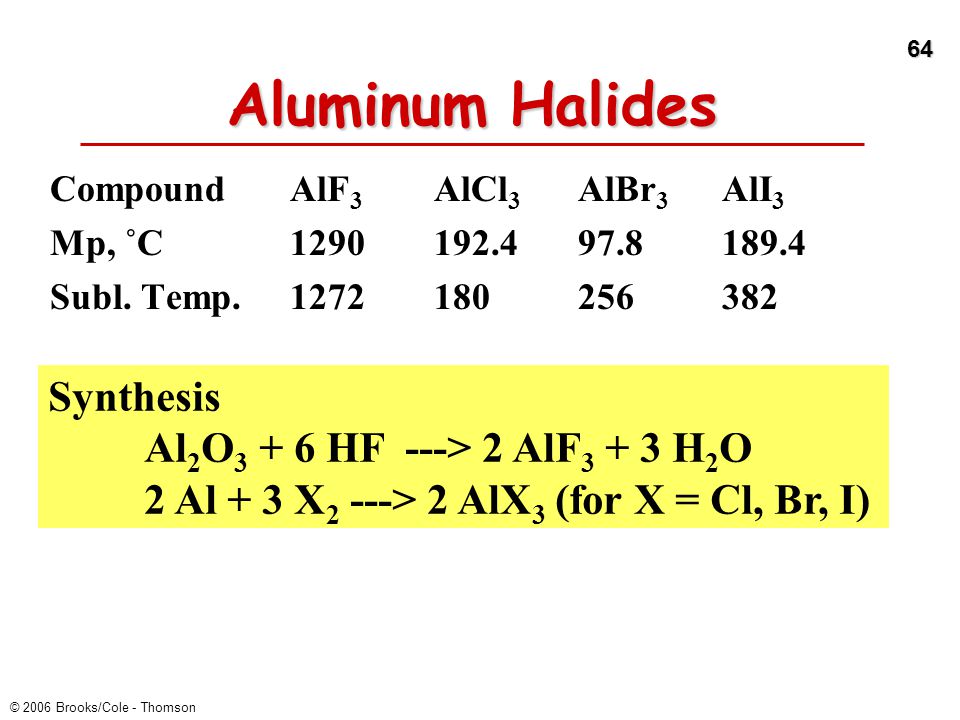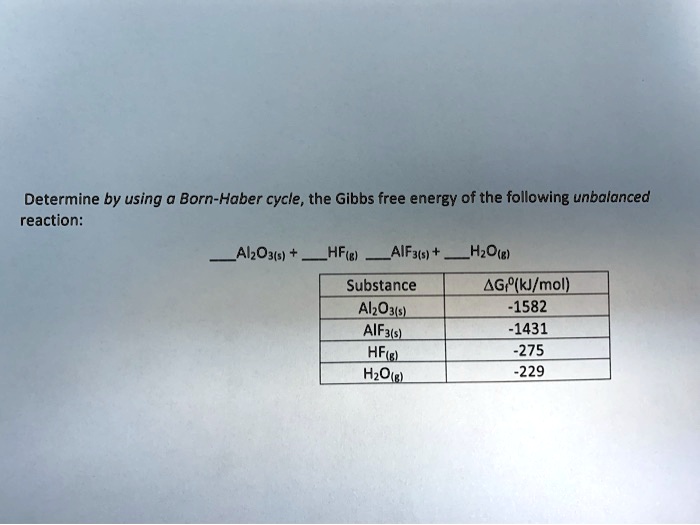
SOLVED: Determine, by using the Born-Haber cycle, the Gibbs free energy of the following unbalanced reaction: Al2O3(s) + 2HF(g) â†' 2AlF3(s) + 3H2O(l) AGr%(kJ/mol) -1582 1431 -275 -229 Substance Al2O3(s) AlF3(s) HF(g)

PDF) Stability of the AlF3 surface in H2O and HF environments: An investigation using hybrid density functional theory and atomistic thermodynamics | Sven Schroeder - Academia.edu
Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/8-Figure4-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar
![PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e2ac2e88133be5a7278abdd433a3dd75d426a246/2-Figure1-1.png)
PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar
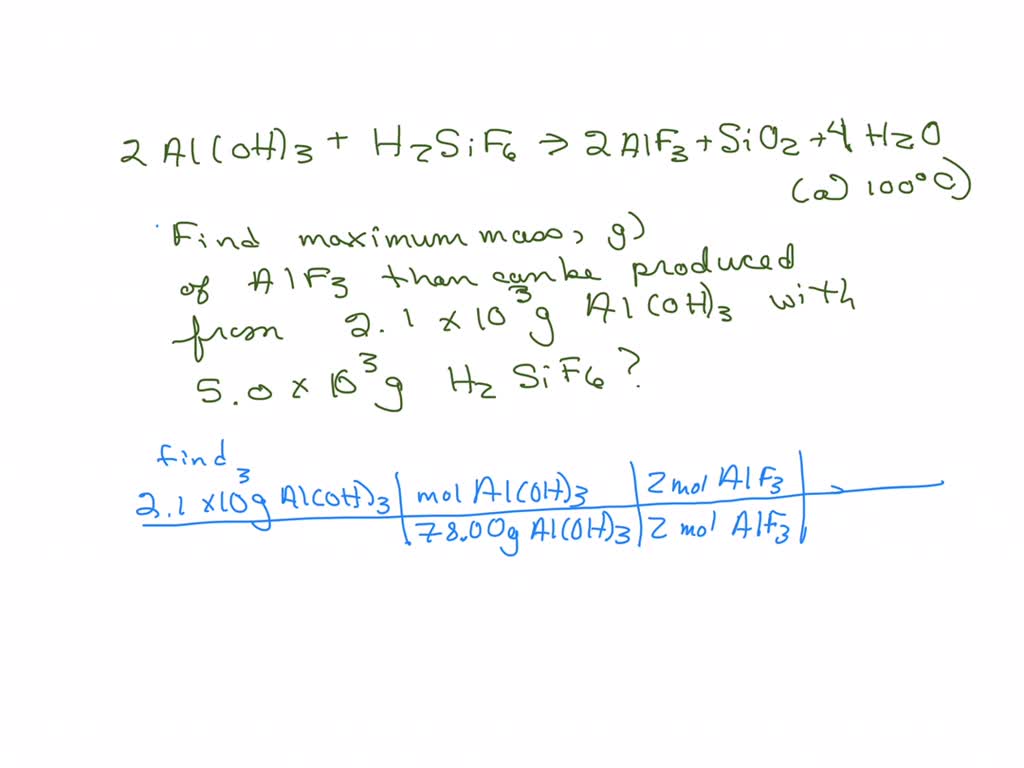
SOLVED: The equation for one process for making aluminum fluoride follows. What is the maximum mass, in grams, of aluminum fluoride, AlF3, that can be produced from the complete reaction of 2.1

Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar

Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment | ACS Applied Energy Materials

Atomic Layer Etching of AlF3 Using Sequential, Self-Limiting Thermal Reactions with Sn(acac)2 and Hydrogen Fluoride | The Journal of Physical Chemistry C

Reaction Mechanisms during Atomic Layer Deposition of AlF3 Using Al(CH3)3 and SF6 Plasma | The Journal of Physical Chemistry C
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/4-Figure1-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar
![The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1293255816303363-fx1.jpg)
The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect

Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar