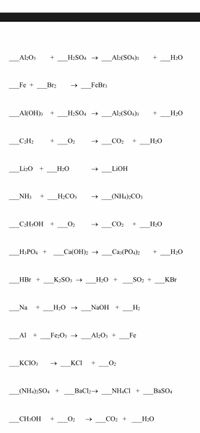
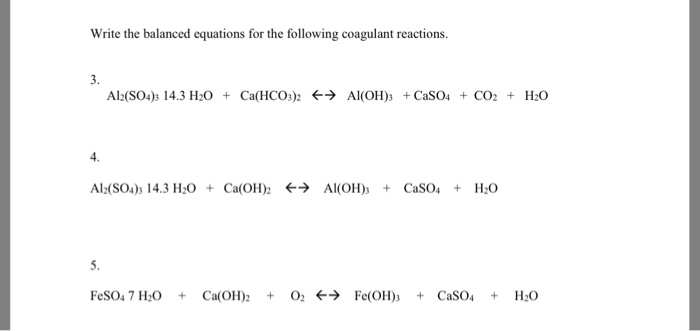
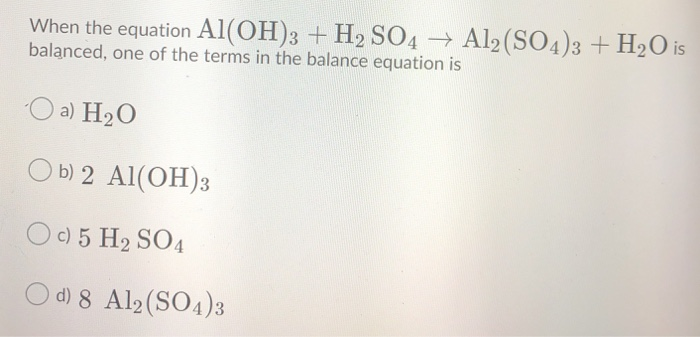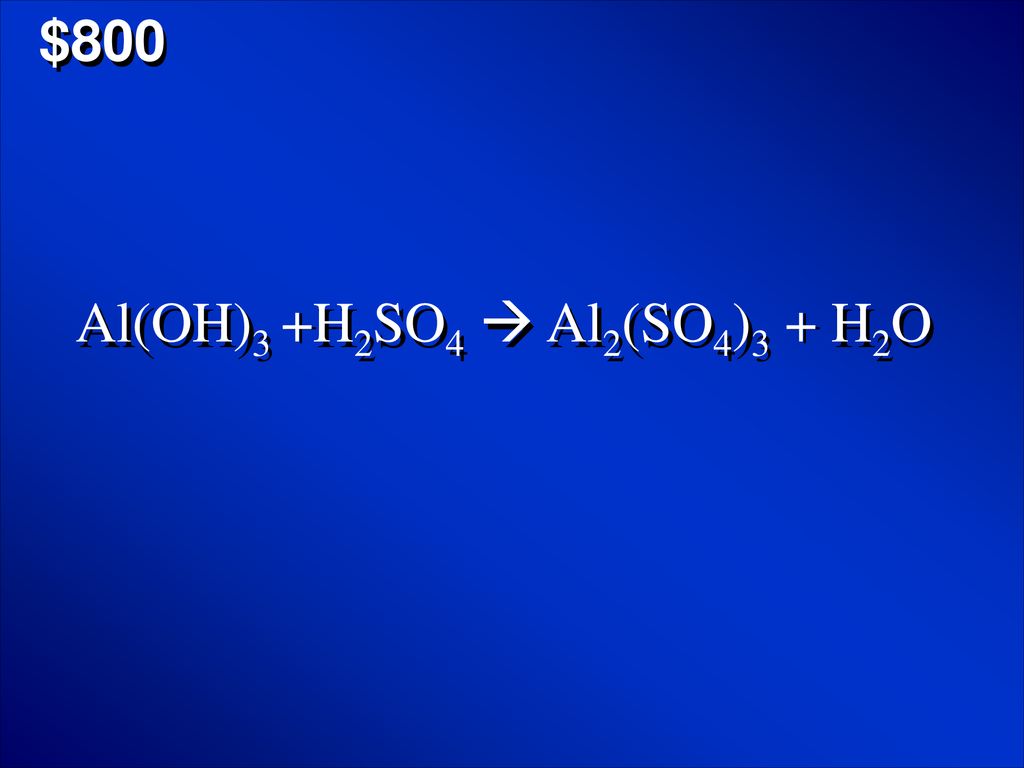Consider the reaction of Al2O3 withH2SO4 to form Al2(SO4)3 and H2O. If 3.84 g AL2O3 is reacted with excess - brainly.com
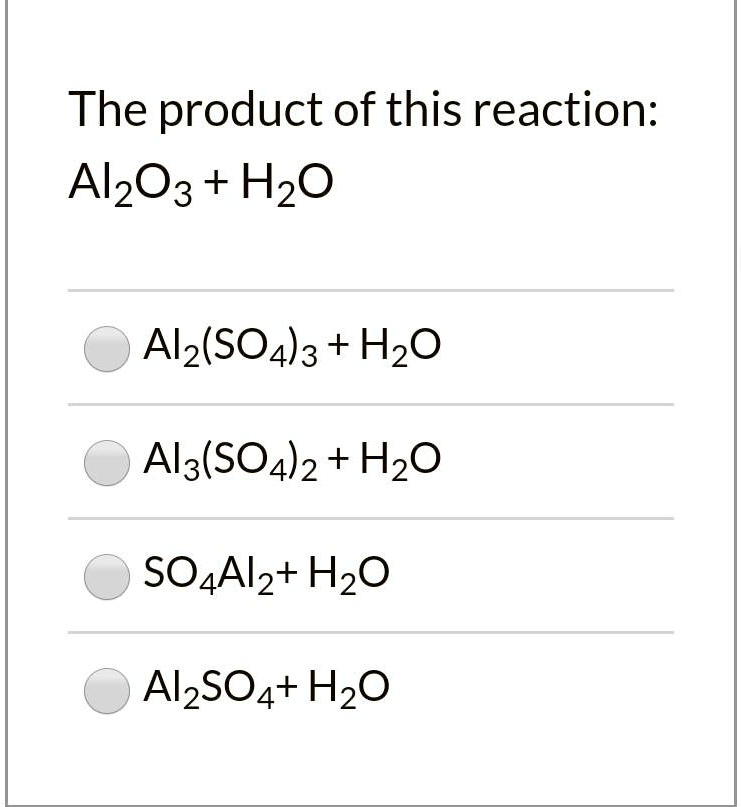
SOLVED: The product of this reaction: Al2O3 + H2O Al2(SO4)3 + H2O Al3(SO4)2 + H2O SO4Al2 + H2O Al2SO4 + H2O

OneClass: Mixing 39.0g of Al(OH)3 with an excess of H2SO4 we produce Al2(SO4)3. Using the following e...

Non-Ferric Aluminium Sulphate (Formula: Al2 (SO4) 3 X H2O (X 14-18) - China Nonferric, Iron Free | Made-in-China.com

Aluminium sulphate solution 0.2 % Al2(SO4)3 * 18 H2O for blue number determination Contents:, 48,91 €

Phase Diagrams of (NH4)2SO4–Al2(SO4)3–H2O Ternary System: Effect of Sulfuric Acid and Its Application in Recovery of Aluminum from Coal Fly Ash | Journal of Chemical & Engineering Data
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3 + NO H2O H2S + K2Cr2O7 + H2SO4 =KHSO4 + Cr2(SO4)3 + S + H2O Balance the equations using ion electron method.
:no_upscale()/3000.jpg)
19516.3000 - Aluminium sulfate solution, 40 % Al2(SO4)3 * 18 H2O/l, technical grade, 1 L | Analytics-Shop

Aluminium sulphate solution 30 % Al2(SO4)3 * 18 H2O for Blue Number determination Contents: 2, 529,55 €

Solved) - Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l) Express your answer... (1 Answer) | Transtutors

Mass and Molar quantities of Ca(OH2) and Al2(SO4)3 · 18 H2O used in the... | Download Scientific Diagram