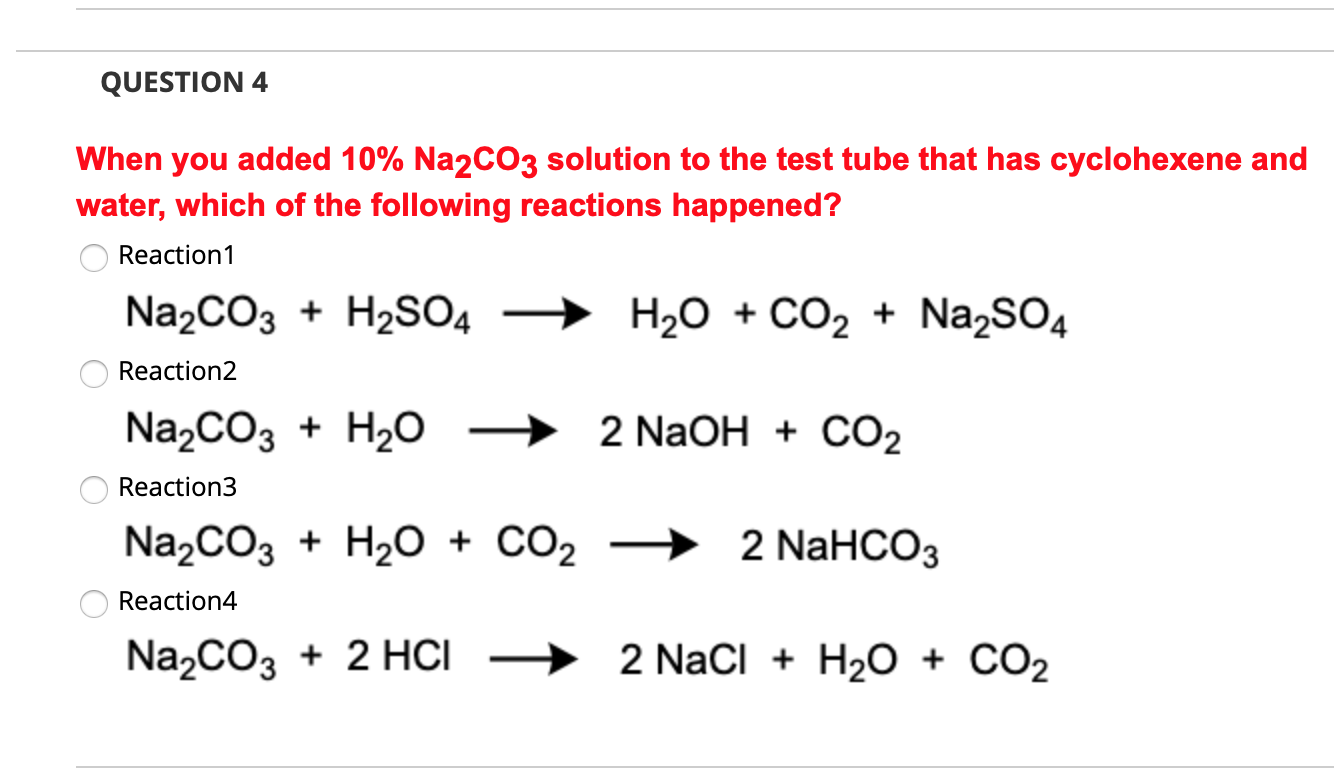
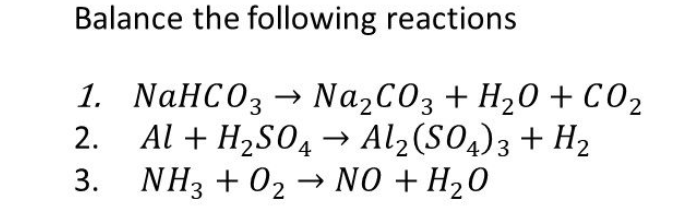NaHCO3 decomposes as : 2NaHCO3(s) Na2CO3(s) + CO2(g) + H2O(g) The equilibrium pressure is 1.04 atm. The Kp the reaction is : (1) 0.2704 (2) 2.704 (3) 27.04 (4) 270.4

How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of b… | Test tube, Solutions, Completed

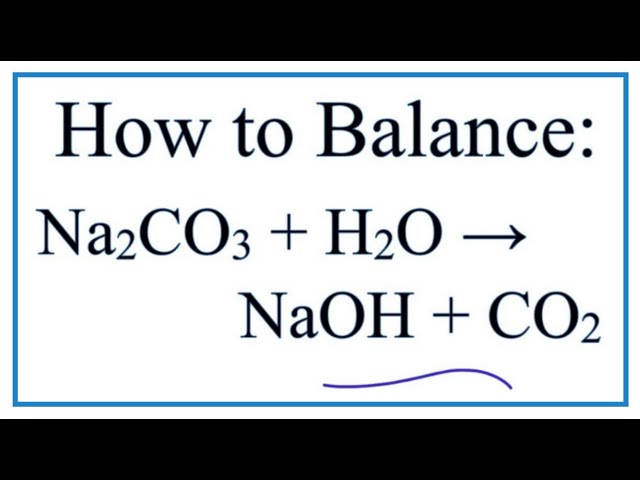
How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Chemistry 360 | Facebook

Scheme 3. Reagents and conditions: a) H2O, Na2CO3, rt, 1 min; b) CH3CN,... | Download Scientific Diagram