Rare and Nonexistent Nitrosyls: Periodic Trends and Relativistic Effects in Ruthenium and Osmium Porphyrin-Based {MNO}7 Complexes | ACS Omega

MnO_(4)^(-)` oxidises `H_(2)O_(2)` to `O_(2)` in acidic medium `xMnO_(4)^(-)+yH_(2)O_(2)+zH^(+) rarr Mn^(2+)+O_(2)+H_(2)O` Coefficients `x`, `y` and - Sarthaks eConnect | Largest Online Education Community

Influence of Common Anions on the Coordination of Metal Cations in Polyalcohols - Teichert - 2019 - European Journal of Inorganic Chemistry - Wiley Online Library

Bac sciences - ✓💡⚠️بعض المزدوجات الهامة تفاعل أكسدة اختزال المستعملة بالسنة الختامية.⚠️ PC&SM&SVT(2bac)✓ #فيزياء_و_كيمياء | Facebook

Highly efficient NH3-SCR of NOx over MnFeW/Ti catalyst at low temperature: SO2 tolerance and reaction mechanism - ScienceDirect

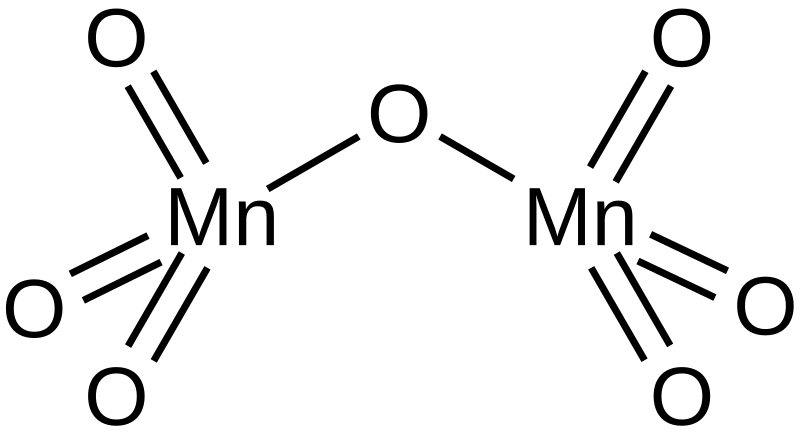
Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega

For the redox reaction MnO2 + C202 +H- Mn2+ + CO2 + H2O the correct coefficients of the reactants the balanced equation are MnO4 C204 H+ (1) 16 5 (2) 2 5 16 (3) 2 (4) 5
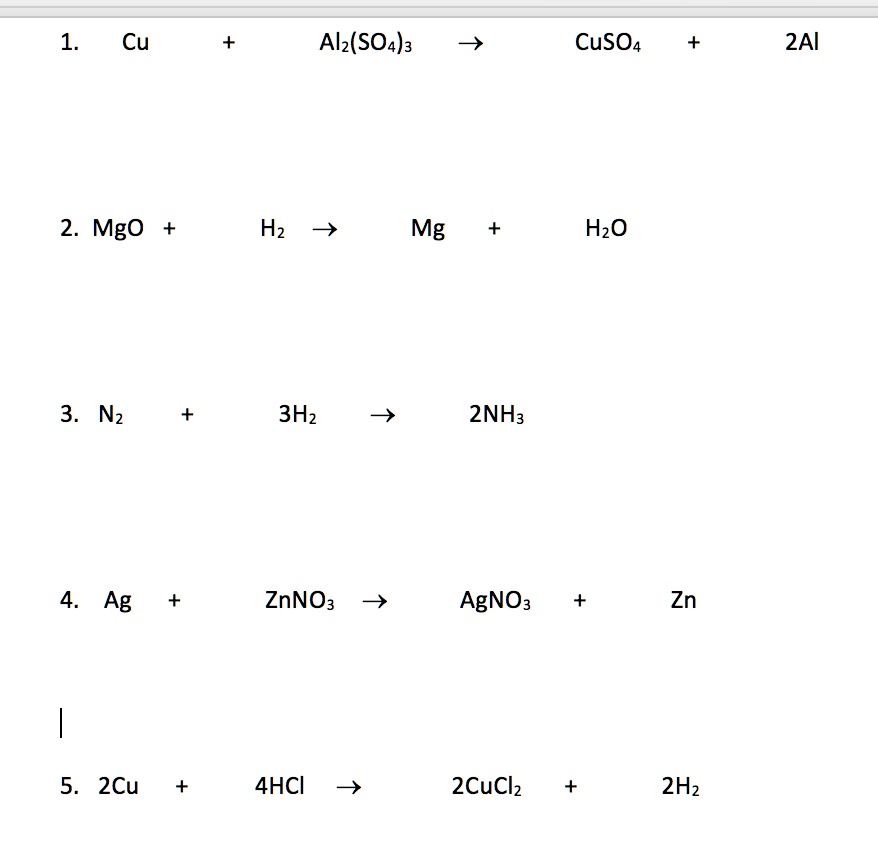
SOLVED: 1. Cu + Al2(SO4)3 â†' CuSO4 + 2Al 2. MgO + H2O â†' Mg + H2O 3. N2 + 3H2 â†' 2NH3 4. Ag + Zn(NO3)2 â†' AgNO3 + Zn 5. 2Cu + 4HCl â†' 2CuCl2 + 2H2

Balance redox reaction by ion electron method or half reaction method. Fe2++MnO4-+H+=Fe3++Mn2++H2O. - YouTube
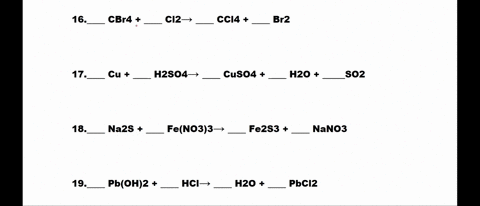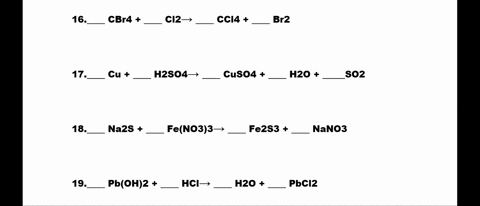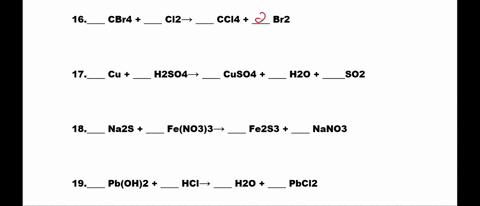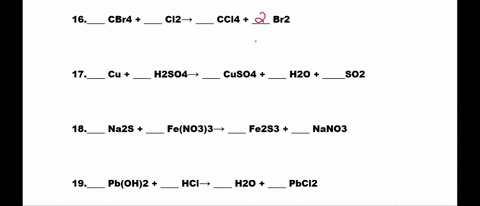
SOLVED: 1. N2 + H2 â†' NH3 2. CH4 + O2 â†' CO2 + H2O 3. P4 + O2 â†' P2O3 4. H2 + NO â†' N2 + H2O 5. Na +

Novel carbohydrate-substituted cyclopentadienyls of titanium, molybdenum, manganese and iron - ScienceDirect

For the redox reaction MnO2 + C202 +H- Mn2+ + CO2 + H2O the correct coefficients of the reactants the balanced equation are MnO4 C204 H+ (1) 16 5 (2) 2 5 16 (3) 2 (4) 5

Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega

Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega