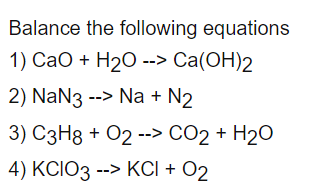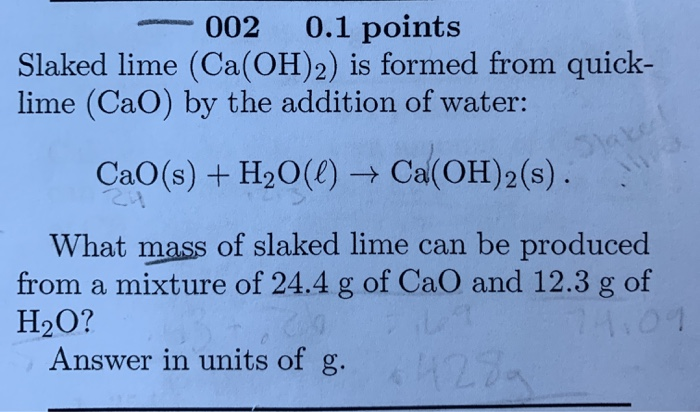Insight into the Mechanism and Effect of H2O on CaO Sulfation by Density Functional Theory | Energy & Fuels
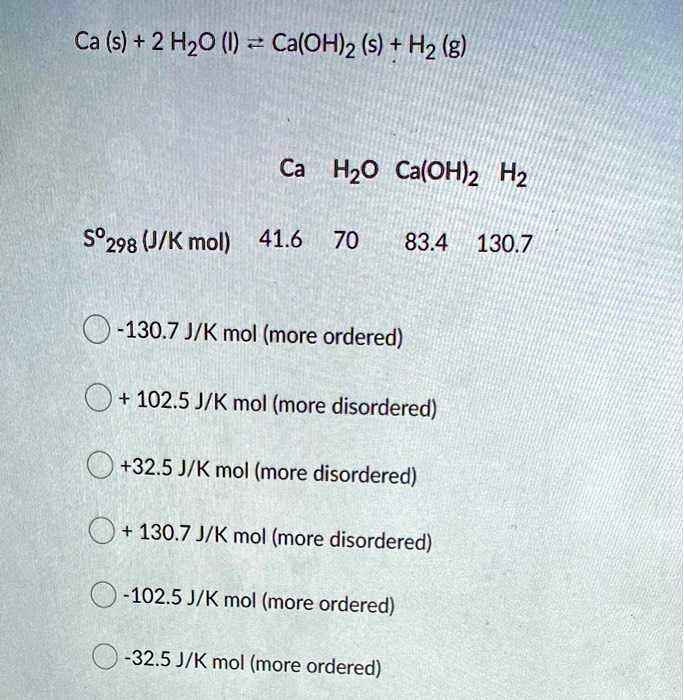
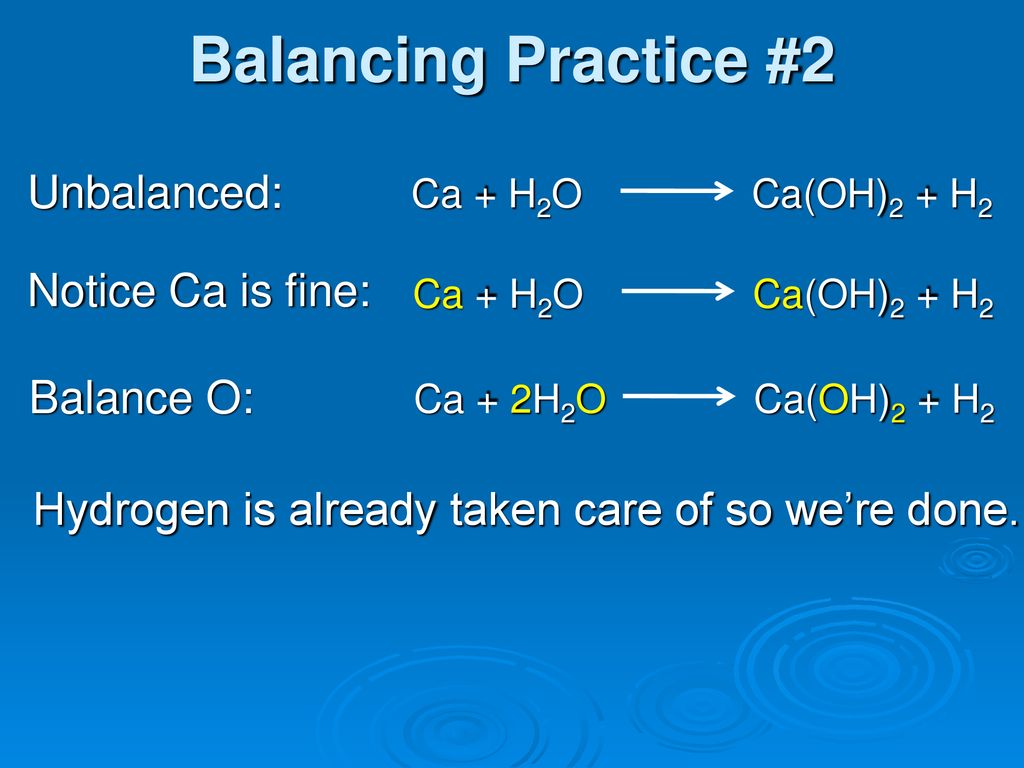
SOLVED: Ca (s) + 2 H2O (l) = Ca(OH)2 (s) + H2 (g) Ca H2O Ca(OH)2 H2 592.98 (J/K mol) 41.6 70 83.4 130.7 413.07 J/K mol (more ordered) 102.5 J/K mol (

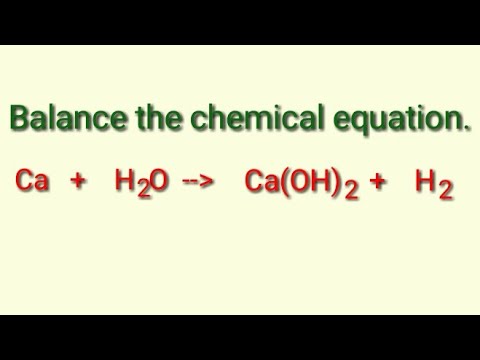
How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) | How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) Hi Guys, welcome back to
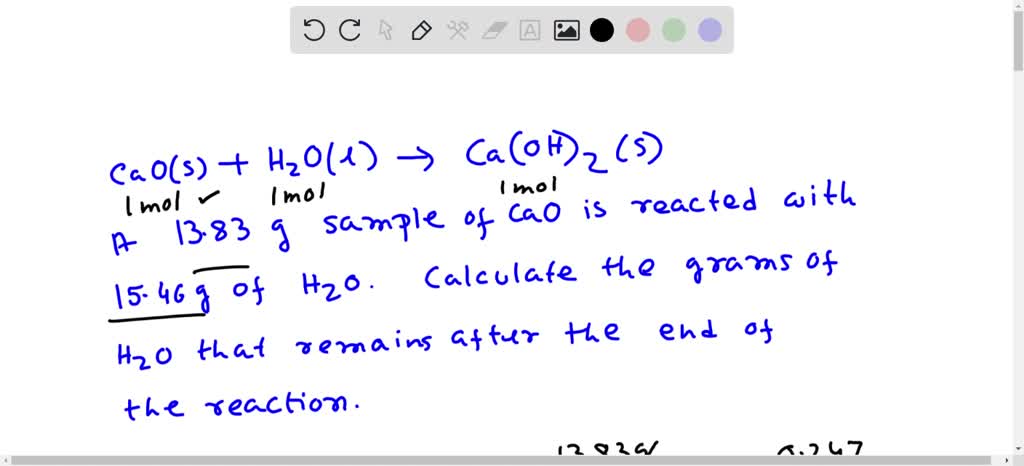
SOLVED: Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + H2O(l) → Ca(OH)2 (s) A 13.83 g sample of CaO is reacted with 15.46 g of

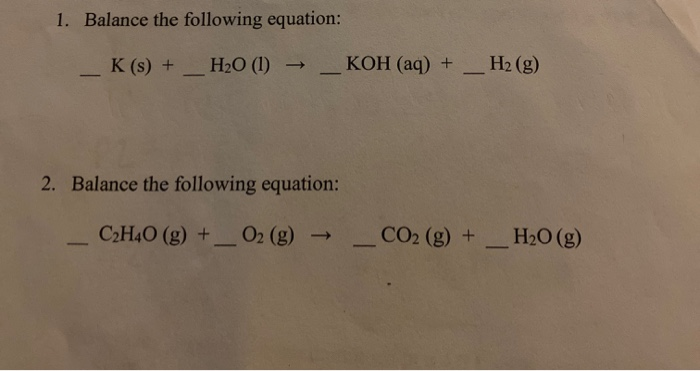
9.24 Complete the following chemical reactions. i) CaO + H2O → ii) AICI, + H2O → iii) Ca N2 (6) + H2O → iv) PbS+ H202 →

Phase diagram for the system Ca(OH)2-CaO-O2.Free energy of the calcium... | Download Scientific Diagram

chemmacros - What is wrong with \ch{CaO$_{(s)} + H2O$_{(l)}->Ca$^{2+}_{(aq)} + 2 OH$^-_{(aq)}} - TeX - LaTeX Stack Exchange