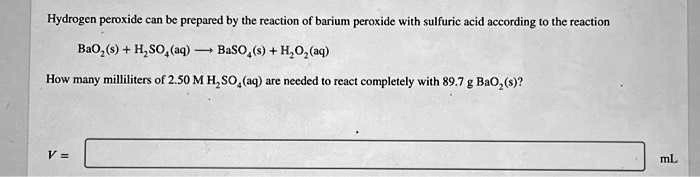
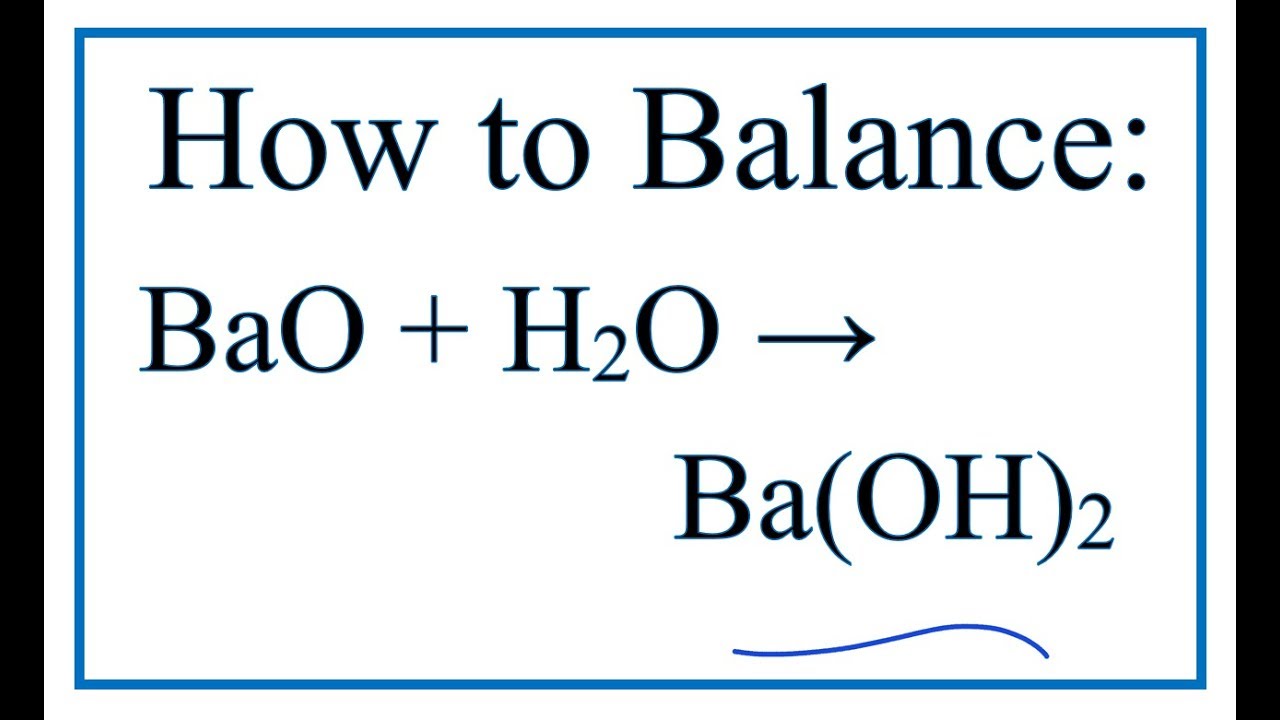
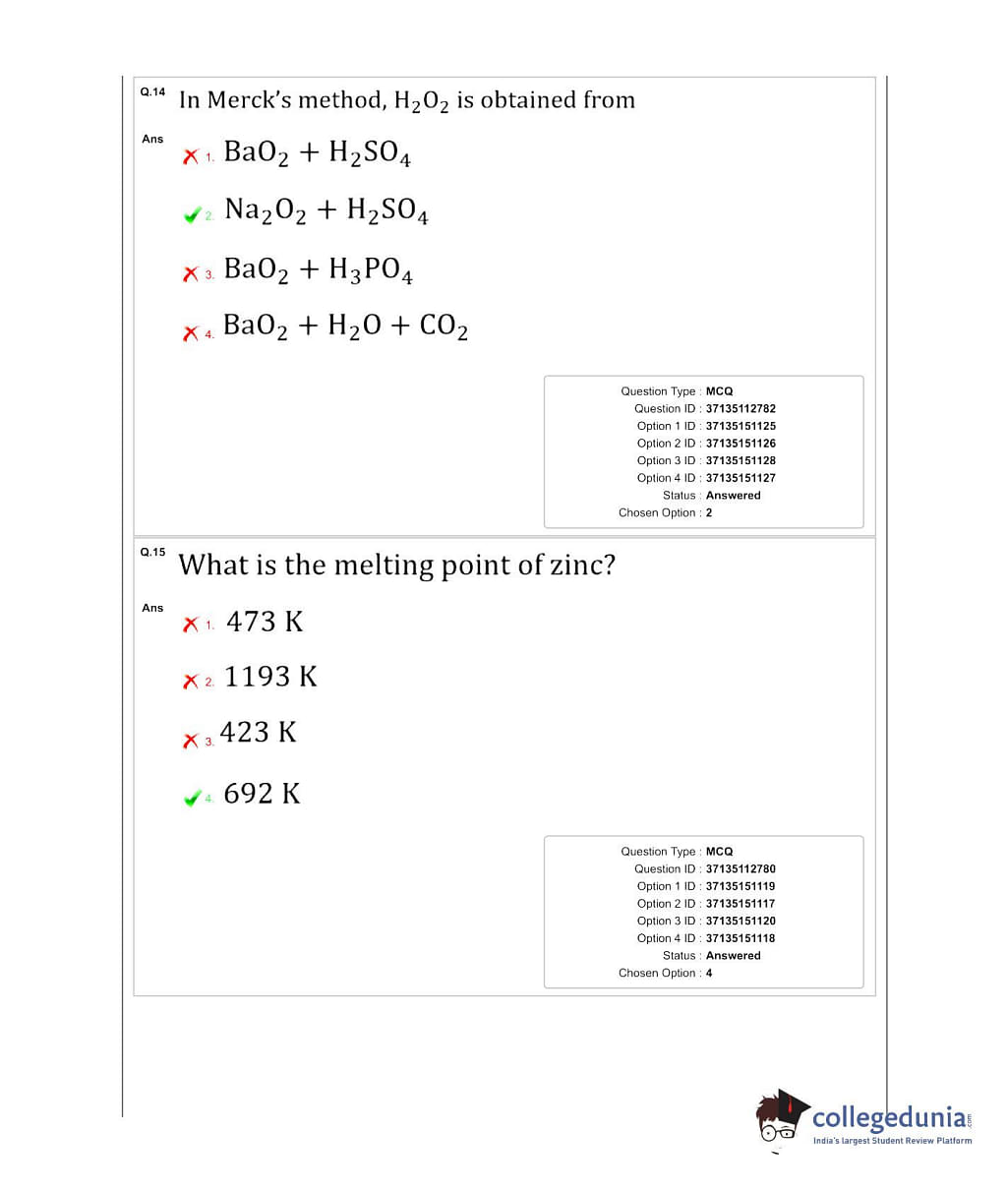
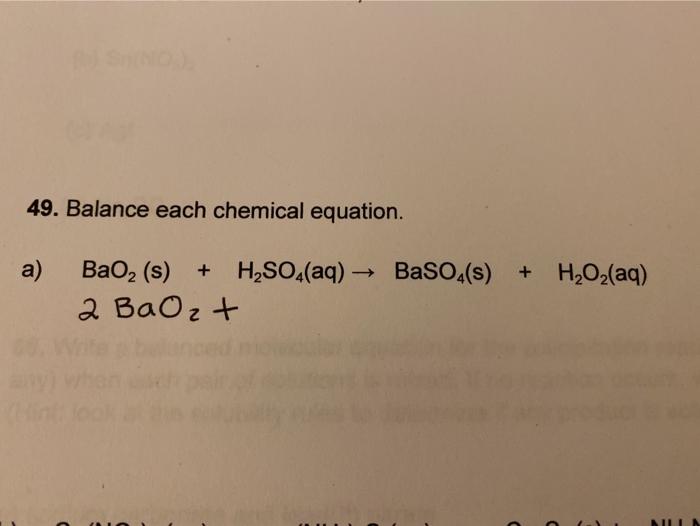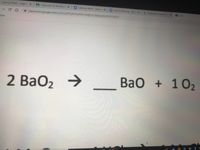What is the oxidation state of the most electronegative element in the products of the reaction between BaO2 and dil.H2SO4? - Quora

07. Which is not a redox reaction? 1) BaO, +H,SO→ BaSO4 + H2O 2) 2BaO +0, 2Bao, 3) 4KCIO, — 4KCIO, + 20, 4) SO, + 2H,S → 2H,0 + 3S

The compound that gives \( \mathrm{H}_{2} \mathrm{O}_{2} \) on treatment with dilute \( \mathrm{... - YouTube
General and inorganic chemistry I: Required knowledge This document describes the scope of knowledge that students are expected

Influence of the total pressure on the thermodynamic equilibrium of... | Download Scientific Diagram

Chemical Reactions: evidence, writing them, balancing them Zumdahl Chapter 6 Notes Start Date: ______. - ppt download