Al(H2O)6 3+ + H2O clusters with (A) single H-bond; (B and C) double... | Download Scientific Diagram

Pompe pour eaux usées - ALH20 - Albin Pump - pour eau industrielle / pour produits chimiques / à boue

Aluminum Alloys 6061 65032 H20 Al-Mg-Si Cu Round Bar at Rs 310/kilogram | Aluminium Alloy Wire in Mumbai | ID: 14138849273
2 Aluminium chloride in acidified aqueoussolution forms a complex A', in whichhybridisation state of Al is 'B. What are A'and 'B, respectively?(a) Al (H20)613, sp^° d(b) Al(H2O)43, sp3(c) Al(H2O)43, dsp2(d) Al(H20)63, d sp
![Isostructural series of [{Al(H2O)6}{Ln(pda)3}].10H2O: Synthesis, structure and photoluminescence - ScienceDirect Isostructural series of [{Al(H2O)6}{Ln(pda)3}].10H2O: Synthesis, structure and photoluminescence - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0020169318313744-gr1.jpg)
![Solved [Al(H20)6]3+ (aq) + OH” (aq) = [Al(H2O); (OH)]2+ (aq) | Chegg.com Solved [Al(H20)6]3+ (aq) + OH” (aq) = [Al(H2O); (OH)]2+ (aq) | Chegg.com](https://media.cheggcdn.com/media/9e1/9e138992-041d-4ce8-af70-b90c4bc015a4/phpSRes8O)


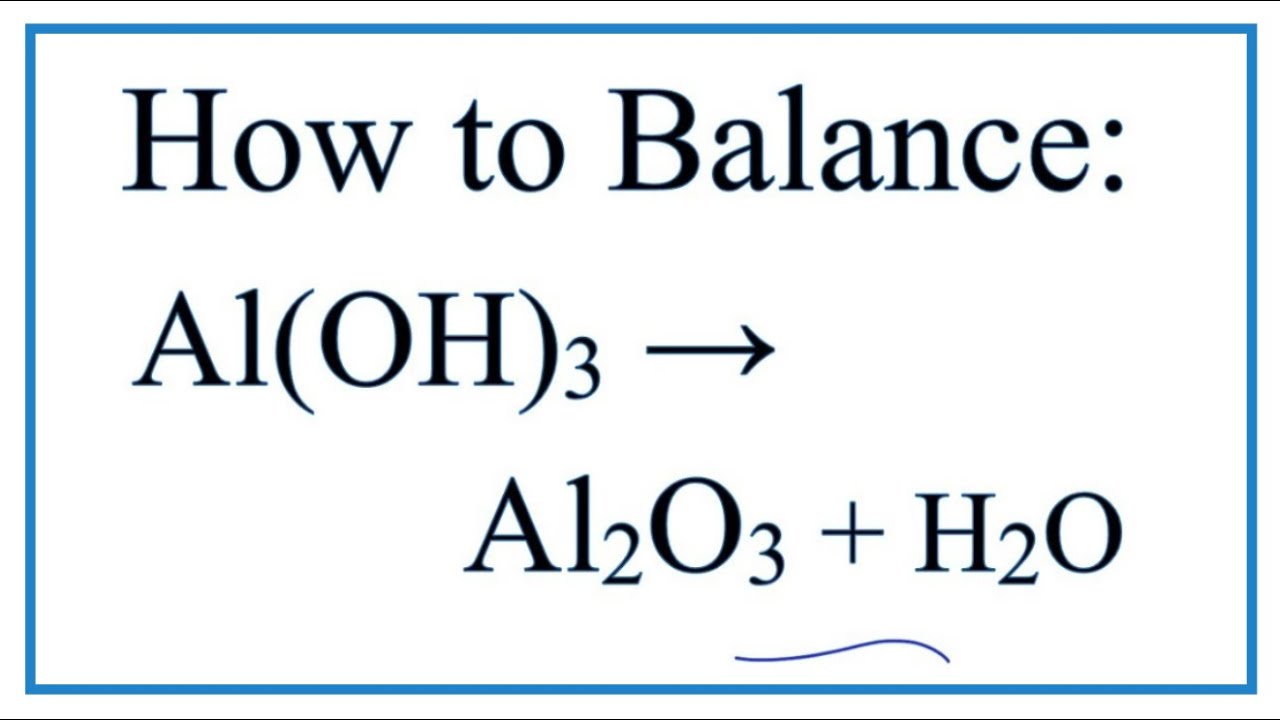
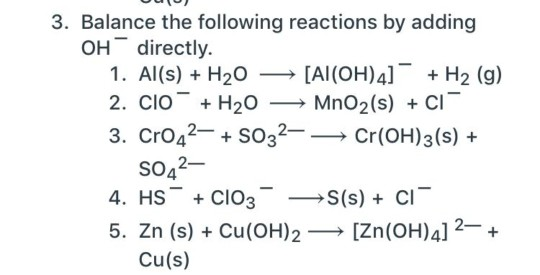

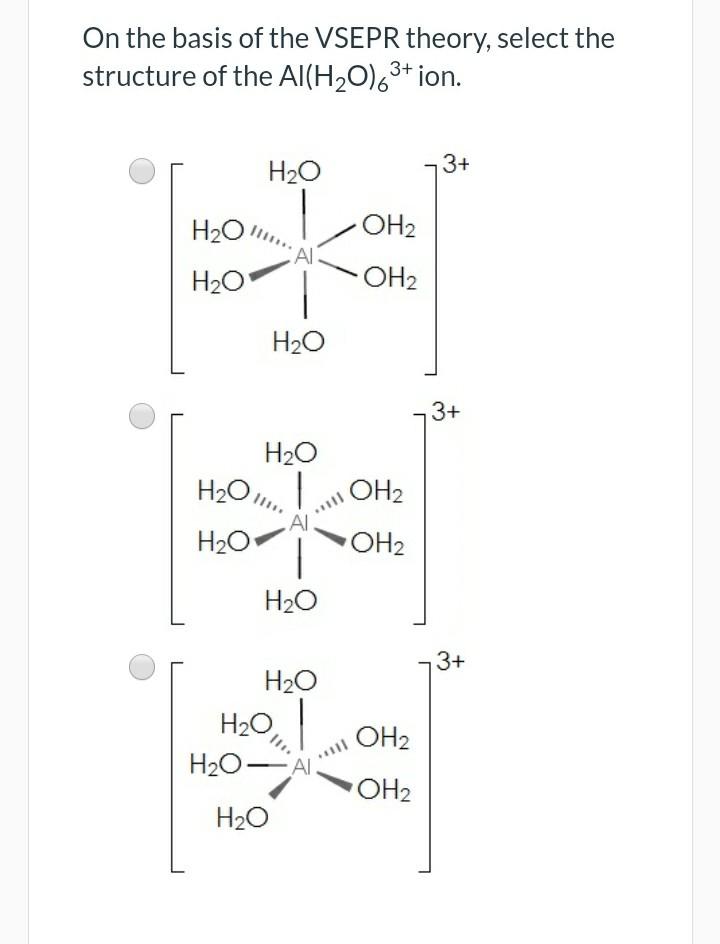
)




![1- In Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Lewis base, Bronsted-Lowry base - YouTube 1- In Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Lewis base, Bronsted-Lowry base - YouTube](https://i.ytimg.com/vi/cBYI3FKTLIo/maxresdefault.jpg)






